0555
Intra-bin correction and inter-bin compensation of respiratory motion in free-running 5D whole-heart MRI1Diagnostic and Interventional Radiology, Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland, 2University of Lausanne, Lausanne, Switzerland, 3Center for Biomedical Imaging (CIBM), Lausanne, Switzerland, 4University Lyon, Lyon, France, 5Radiology, Louis Pradel Hospital, Lyon, France, 6Service of Cardiology, Heart and Vessel Department, Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland, 7Division of Pediatric Cardiology, Woman-Mother-Child Department, Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland, 8Advanced Clinical Imaging Technology, Siemens Healthcare International AG, Lausanne, Switzerland, 9IHU LIRYC, Electrophysiology and Heart Modeling Institute, Université de Bordeaux, Bordeaux, France, 10Department of Cardiovascular Imaging, Hôpital Cardiologique du Haut-Lévêque, Bordeaux, France
Synopsis
Keywords: Heart, Motion Correction
In this work, we develop, validate, and apply a novel reconstruction framework for intra-bin correction and inter-bin compensation of respiratory motion in cardiac and respiratory motion-resolved free-running whole heart 5D MRI. We demonstrate respiratory resolved images with reduced artifact and without compressing the underlying physiological motion providing a means to increase the acquired resolution, accelerate the acquisition, or investigate respiratory driven changes in cardiac output and blood flow, potentially leading to new MRI driven biomarkers for cardiovascular diseaseBackground
Cardiac and respiratory motion-resolved whole-heart 5D imaging has been shown to improve acquisition efficiency1,2 while also providing a means of evaluating respiratory driven changes in cardiac output and blood flow3. However, two limitations of respiratory-resolved imaging exist which are related to user-defined parameters. First, the number of reconstructed respiratory phases presents a trade-off between the level of undersampling, which may lead to residual artifact, and the amount of respiratory motion within each reconstructed phase (intra-bin), which may lead to image blur. Second, a weighting parameter in the reconstruction must be chosen such that undersampling artifact is reduced without introducing blur due to the remaining motion between each reconstructed phase (inter-bin). Overall, these parameters may be subject-specific and may limit our achievable resolution and acceleration factor.In this work, we develop and validate strategies for intra- and inter-bin respiratory motion compensation to improve the quality of whole-heart 5D MRI in terms of sharpness, residual artifact level, and fidelity of the underlying respiratory motion1,2,4. We validate our approach using a comprehensive numerical simulation and further compare reconstructions with and without the proposed motion compensation strategies in a cohort of 16 patients. With the goal of an improved reconstruction that may enable increased resolution or a reduction in scan time, we test the hypotheses that the inclusion of non-rigid deformation fields in the image reconstruction will enable larger regularization weights to reduce artifact without incurring blur and that the combined approach for intra- and inter-bin respiratory motion compensation will result in improved image quality for whole-heart 5D MRI.
Methods
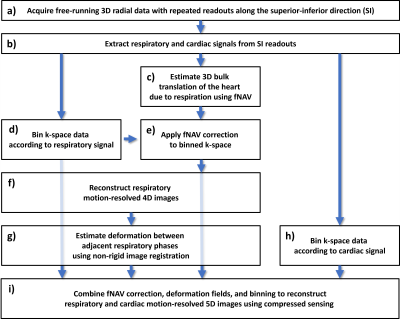
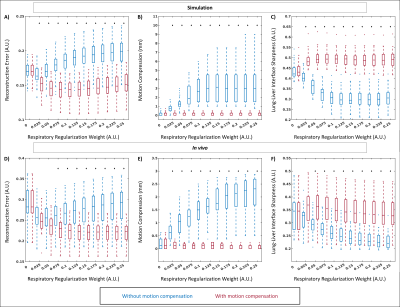
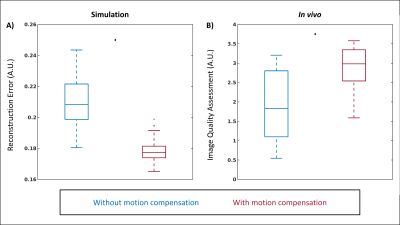
For our proposed reconstruction framework (Fig. 1), we adapted a previously established method (fNAV) for estimating respiratory motion in 3D radial data5 to correct the motion within the bins (intra-bin) of our reconstructions. Additionally, we estimated nonrigid deformation fields between adjacent frames (inter-bin)6. Finally, we combined the estimated intra-bin correction and inter-bin compensation with cardiac and respiratory self-gating to bin the data and reconstruct 5D images2. To validate this framework, a numerical simulation of free-running 3D radial data was developed5,7,8. Simulated data (N=50), each with variable respiratory motion amplitude and heart rate, were generated to match in vivo sequence parameters. To demonstrate the feasibility of our approach in vivo, 16 patients with congenital heart disease were included in this IRB approved study. Examinations were performed without sedation, during free breathing, on a 1.5T clinical MRI system (MAGNETOM Sola, Siemens Healthcare, Erlangen, Germany) after administration of ferumoxytol. A slab-selective spoiled gradient echo free-running 3D radial research sequence4 was used and resulted in uninterrupted acquisitions of six minutes1,6. Relevant parameters were RF excitation angle: 15°, resolution: (1.15 mm)3, FOV: (220 mm)3, TE/TR: 1.53/2.84 ms, readout bandwidth: 1002 Hz/pixel. To test their impact on reconstructions with and without motion compensation, respiratory regularization weights were varied between 0 and 0.25 and the resulting images from both simulated and in vivo data were compared. Reconstruction error (root mean squared difference) over a region of interest containing the lung-liver interface was calculated using the ground truth as reference for simulation and a reconstruction containing all the cardiac bins and without compressed sensing for in vivo data. Compression of respiratory motion was quantified by measuring the displacement of the liver relative to the reference images. Sharpness of the lung-liver interface was quantified as the slope of a sigmoid function fitted to the interface9. Using the optimum regularization weight in terms of these three metrics, 5D images from all simulated and in vivo datasets were reconstructed and the error over a region containing the heart was quantified in simulation data where ground truth is known, and the image quality of in vivo data was assessed using an artificial-intelligence based algorithm trained to grade 3D radial images of the heart10. Statistical significance was measured using a paired t-test.Results
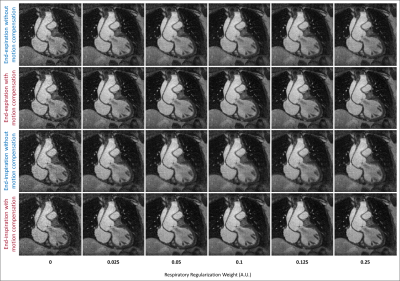
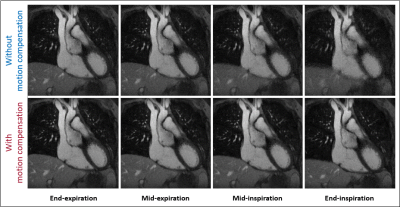
Reconstruction error, motion compression, and sharpness measurements from simulated (Fig. 2a-c) and in vivo (Fig. 2d-e) data demonstrate a consistent statistically significant improvement across the majority of the tested regularization weights when using motion compensation. In vivo image reconstructions (Fig. 3) corroborate the quantitative results from Fig. 2 and display the improved image quality provided by the proposed method, despite increasing regularization weights. For both simulated (Fig 4a) and in vivo (Fig. 4b) data, metrics for image quality are significantly higher using motion compensation. Finally, an animated depiction of 5D images (Fig. 5) using the optimized parameters shows a clear improvement in image quality, particularly during the mid and end-inspiration phases.Discussion and Conclusions
A novel framework for respiratory motion compensation was developed, validated, and applied to whole heart 5D MRI in 16 patients. Quantitative assessment of image quality yielded statistically significant improvements when using the proposed framework while in vivo findings corroborated the results from numerical simulations. Overall, this work not only highlights potential sources of error when reconstructing respiratory resolved images without compensation but provides a robust solution that enables future studies that aim to increase the achievable resolution, decrease acquisition time, and investigate respiratory driven changes in cardiac output and blood flow, potentially leading to new MRI driven biomarkers for cardiovascular disease.Acknowledgements
MS is the PI on the Swiss National Science Foundation grants 320030_173129 and 201292 that funded part of this research. CWR is the PI on Swiss National Science Foundation Grant PZ00P3_202140 that funded part of this research.References
1. Feng L, Coppo S, Piccini D, et al. 5D whole-heart sparse MRI. Magn. Reson. Med. 2018;79:826–838 doi: 10.1002/mrm.26745.
2. Di Sopra L, Piccini D, Coppo S, Stuber M, Yerly J. An automated approach to fully self-gated free-running cardiac and respiratory motion-resolved 5D whole-heart MRI. Magn. Reson. Med. 2019;82:2118–2132 doi: 10.1002/mrm.27898.
3. Ma LE, Yerly J, Piccini D, et al. 5D Flow MRI: A Fully Self-gated, Free-running Framework for Cardiac and Respiratory Motion-resolved 3D Hemodynamics. Radiol. Cardiothorac. imaging 2020;2:e200219 doi: 10.1148/ryct.2020200219.
4. Roy CW, Di Sopra L, Whitehead KK, et al. Free-running cardiac and respiratory motion-resolved 5D whole-heart coronary cardiovascular magnetic resonance angiography in pediatric cardiac patients using ferumoxytol. J. Cardiovasc. Magn. Reson. 2022;24:39 doi: 10.1186/s12968-022-00871-3.
5. Roy CW, Heerfordt J, Piccini D, et al. Motion compensated whole-heart coronary cardiovascular magnetic resonance angiography using focused navigation (fNAV). J. Cardiovasc. Magn. Reson. 2021;23:33 doi: 10.1186/s12968-021-00717-4.
6. Asif MS, Hamilton L, Brummer M, Romberg J. Motion-adaptive spatio-temporal regularization for accelerated dynamic MRI. Magn. Reson. Med. 2013;70:800–812 doi: 10.1002/mrm.24524.
7. Roy CW, Marini D, Segars WP, Seed M, Macgowan CK. Fetal XCMR: a numerical phantom for fetal cardiovascular magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 2019;21:29 doi: 10.1186/s12968-019-0539-2.
8. Wissmann L, Santelli C, Segars WP, Kozerke S. MRXCAT: Realistic numerical phantoms for cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2014;16:63 doi: 10.1186/s12968-014-0063-3.
9. Ahmad R, Ding Y, Simonetti OP. Edge sharpness assessment by parametric modeling: Application to magnetic resonance imaging. Concepts Magn. Reson. Part A 2015;44:138–149 doi: 10.1002/cmr.a.21339.
10. Piccini D, Demesmaeker R, Heerfordt J, et al. Deep Learning to Automate Reference-Free Image Quality Assessment of Whole-Heart MR Images. Radiol. Artif. Intell. 2020;2:e190123 doi: 10.1148/ryai.2020190123.
Figures