0552
Real Time Cardiovascular MR During Exercise in Heart Failure with Preserved Ejection Fraction (HFpEF)1Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Medicine, Cardiovascular Division, University of Wisconsin-Madison, Madison, WI, United States, 3Radiology, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Keywords: Heart, Cardiovascular, Exercise, real time
Right heart catheterization during exercise is the current gold standard for diagnosis of heart failure with preserved ejection fraction (HFpEF) but carries the risk of an invasive procedure. In this work, we present preliminary data using real time cardiac magnetic resonance imaging during exercise in a HFpEF cohort with comparison to healthy controls.
Introduction
Right heart catheterization (RHC) is the gold standard for diagnosis of heart failure with preserved ejection fraction (HFpEF) but is an invasive procedure. Cardiac magnetic resonance (CMR) is non-invasive and is the gold standard for measures of global cardiac function. Robust cardiac MRI requires breath holds, which may conceal symptoms, and cine reconstructions, which require good cardiac gating and constant heart rate. Submaximal physiologic exercise has been suggested to phenotype HFpEF1 but is difficult in the MR bore. Exercise compromises gating and exacerbates motion artifacts, impairing cine reconstructions. Exercise CMR in healthy controls is feasible and shows value.2 We hypothesize that real time (RT) CMR exercise imaging can offer a noninvasive alternative to RHC in assessing HFpEF. Here we present preliminary cardiac MRI data obtained with a newly implemented realtime acquisition and reconstruction pipeline, comparing data from controls with HFpEF patients at submaximal exercise.Methods
Volunteers were imaged on a 3T scanner (Discovery MR750, GE Healthcare, Waukesha, WI) with an 8-channel cardiac coil at rest and during exercise with a pneumatic MRI-compatible exercise stepper (Cardio Step Module; Ergospect, Innsbruck, Austria) with stepping resistance automatically adjusted to maintain a target exercise power. The healthy controls exercised at 50W, while patients referred from RHC exercised at 25-50% of the VO2 max for an average of 38W. All subjects exercised for 5 minutes to reach steady state heart rate (HR) before imaging.Short axis slices were acquired across the whole heart during rest and exercise. Patients were scanned at rest using a breath held bSSFP cine product sequence reconstructed at 40 cardiac phases: TR/TE/dz=3.3 ms,1.27ms,7mm, spatial resolution=0.78x0.78mm2. A 2D multislice radially undersampled RT sequence was used for realtime imaging: 3,000 projections acquired continuously with 326 samples per spoke and a 0.75 fractional echo readout: TR/TE/dz=2.9 ms,1.1ms,8mm. Radial RT acquisitions were reconstructed using 2 methods: (1)Bellows/ECG gating-based cine binning and (2)ungated RT reconstruction. After binning projections for RT reconstructions with 10 spokes/frame (dt=29 ms), images were reconstructed to a matrix of 160x160 for a spatial resolution=2.25x2.25mm2 using parallel imaging and compressed sensing (with temporal total variation and spatial wavelet L1-norm penalties) via the BART3 toolbox, using coil sensitivity maps determined by ESPIRiT4. The penalty weights were qualitatively chosen: λTV=0.008 and λwavelet=0.004.End diastole (ED) and end systole (ES) frames for both the right ventricle (RV) and left ventricle (LV) were manually segmented and used to calculate ejection fractions (EF). Cine reconstructions were analyzed in Segment,5 while expiration RT ED/ES frames were identified visually and segmented in the ImageJ6 segmentation editor. In addition, RT RV EF obtained with MRI and with catheter lab PV loop data were compared for rest and during exercise.
Results
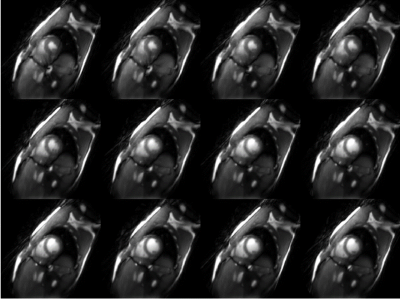
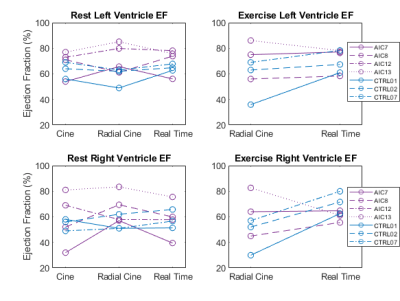
HFpEF patient (n=4) HR rose from 61±7 to 81±10 BPM with exercise while healthy control (n=3) HR rose from 67±3 to 83±7 BPM. An example of RT reconstruction from systole to diastole during exercise is presented in Figure 1. Comparison of the LV and RV EF from the clinical cine versus radial cine (RV mean error =8.4±16%) and radial RT reconstructions (RV mean error = 0.3±9.6%) at rest are shown in Figure 2.RV EF increased with exercise in all three controls (average increase=17±9%) but remained relatively constant in the HFpEF patients (2±17%).
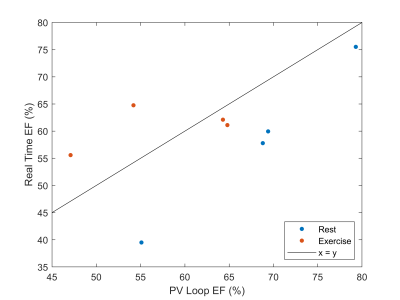
Finally, a comparison of RT RV EF with catheter lab PV loop data is shown at rest and during exercise in Figure 3, showing the RT approach may underestimate EF at rest.
Discussion
While the ejection fractions varied between the clinical standard and rest radial reconstructions, error was lower comparing clinical standard to the RT reconstruction. The radial cine error is due to poor gating during exercise, resulting in improperly binned data. While statistical evaluation of the reported RV EF trend with exercise requires larger n, it is known that EF should increase with exercise in the healthy heart.7 RV EF was also recently found to be significant in a larger (n=68) study of HFpEF versus noncardiac dyspnea at rest.8While cine reconstructions without reliable gating leads to underestimation of EF due to improperly binned projections, lower SNR RT reconstructions may lead to overestimation of EF due to difficulty visualizing blood pool volume in apical slices at end systole. Our cine reconstructions were less reliable especially during exercise due to poor gating quality, caused by the magnetohydrodynamic effect9 or loose leads distorting the ECG trace. We plan to investigate ‘self-gating’ options to enable better cine binning to compare with the RT reconstructions. RT CMR imaging produces hundreds of images – an automated approach to segmentation would be invaluable. We are in the process of testing a CNN for ventricle segmentation,10 which could enable analysis of beat-to-beat variations. The RT approach may underestimate PV loop EF at rest, but PV loop measurements are not perfect. Further study is needed.
Conclusion
In this study, we presented preliminary RT CMR exercise imaging data from HFpEF patients and healthy controls during submaximal exercise. While sample size is low, an increase in EF with exercise appears to exist in the healthy controls but not in the HFpEF patients. Future work will increase number of subjects, compare with RHC measures, and analyze RT flow in the major vessels.Acknowledgements
The University of Wisconsin-Madison receives research support from GE Healthcare. This study was supported by the UW-Madison Department of Medicine pilot fund (233-AAH9756) and the UW-Madison Department of Radiology Research and Development fund.References
1. Pandey A, Shah SJ, Butler J, et al. Exercise Intolerance in Older Adults with Heart Failure with Preserved Ejection Fraction: JACC State-of-the-art Review. J Am Coll Cardiol. 2021;78(11):1166. doi:10.1016/J.JACC.2021.07.014
2. Beaudry RI, Samuel TJ, Wang J, Tucker WJ, Haykowsky MJ, Nelson MD. Exercise cardiac magnetic resonance imaging: A feasibility study and meta-analysis. Am J Physiol - Regul Integr Comp Physiol. 2018;315(4):R638-R645. doi:10.1152/ajpregu.00158.2018
3. Ueker et. al. BART Toolbox. Accessed December 18, 2020. https://mrirecon.github.io/bart/
4. Uecker M, Lai P, Murphy MJ, et al. ESPIRiT - An eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn Reson Med. 2014;71(3):990-1001. doi:10.1002/mrm.24751
5. Heiberg E, Sjögren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of Segment-freely available software for cardiovascular image analysis. Published online 2010. Accessed November 6, 2022. http://segment.heiberg.se.
6. Rueden CT, Schindelin J, Hiner MC, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 2017;18(1):1-26. doi:10.1186/S12859-017-1934-Z/FIGURES/7
7. Li YY, Zhang P, Rashid S, et al. Real-time exercise stress cardiac MRI with Fourier-series reconstruction from golden-angle radial data. Magn Reson Imaging. 2021;75:89-99. doi:10.1016/j.mri.2020.10.010
8. Backhaus SJ, Lange T, George EF, et al. Exercise Stress Real-Time Cardiac Magnetic Resonance Imaging for Noninvasive Characterization of Heart Failure With Preserved Ejection Fraction: The HFpEF-Stress Trial. Circulation. 2021;143(15):1484-1498. doi:10.1161/CIRCULATIONAHA.120.051542
9. Abi-Abdallah D, Robin V, Drochon A, Fokapu O. Alterations in human ECG due to the MagnetoHydroDynamic effect: a method for accurate R peak detection in the presence of high MHD artifacts. Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Int Conf. 2007;2007:1842-1845. doi:10.1109/IEMBS.2007.4352673
10. Bai W, Sinclair M, Tarroni G, et al. Automated cardiovascular magnetic resonance image analysis with fully convolutional networks. arXiv. 2017;20(1):65. doi:10.1186/s12968-018-0471-x
Figures