0544
4D aortic motion maps from isotropic high-resolution 3D CINE balanced steady state free precession at 3T and automated segmentations1Radiology and Nuclear Medicine, Amsterdam UMC location University of Amsterdam, Amsterdam, Netherlands, 2Atherosclerosis and Ischemic Syndromes, Amsterdam Cardiovascular Sciences, Amsterdam, Netherlands, 3Biomedical Engineering and Physics, Amsterdam UMC location University of Amsterdam, Amsterdam, Netherlands
Synopsis
Keywords: Vessel Wall, Segmentation, 4D motion, aorta
In this study we introduce 4D aortic displacement and diameter change estimations from isotropic high-resolution 3D CINE CMR as novel biomarkers for aortic stiffness. These could potentially be used as an alternative to pulse wave velocity stiffness measurements which can be unreliable for very stiff aortas as found in for example Marfan syndrome patients. Using deep learning for segmentation, visualization and quantification of aortic motion is demonstrated through 4D aortic motion maps. Time curves of displacement showed the typical cyclic behavior of cardiac motion, with a maximum displacement median of 10.3 (3.6) mm over 14 healthy volunteers.
Introduction
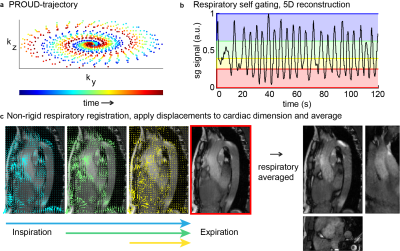
Aortic motion has been shown to have a direct impact on the mechanical stresses acting on the aorta1. Large aortic displacement may be tolerated in a compliant aorta but could be the cause of dissection or rupture in a patient with stiffer aortic tissue. In very stiff aortas in for example Marfan syndrome, the time difference between flow curves needed for pulse wave velocity calculations may be difficult to assess. In pursuit of novel and improved biomarkers for aortic stiffness, this study aimed to derive detailed 4D aortic motion maps and investigate the reproducibility of quantified motion metrics. This was accomplished through free-breathing respiratory-motion-corrected 3D cine of the whole aorta paired with a deep learning method for automated aortic segmentations over the cardiac cycle.Methods
Fourteen healthy control subjects (6 women, 27 ± 5 [25-44] years) underwent three MRI examinations at 3T (Ingenia, Philips, Best, NL) separated by 2-5 minutes (test, retest) and 2 weeks (rescan). The entire thoracic aorta was imaged using a free-running 3D CINE balanced steady state free precession (bSSFP) scan with retrospective cardiac binning and respiratory binning and correction (Figure 1)2. Scan parameters were: isotropic spatial resolution of (1.6 mm)3, FOV: 256x256 x88 mm3, TE/TR: 1.7/3.4 ms, α =30°, scan time of ~3min:48s and reconstructed temporal resolution of ~67msec (15 cardiac frames). Additionally, a 2D CINE bSSFP scan was planned orthogonal to the aortic arch between the brachiocephalic and left common carotid artery with scan parameters: acq./rec. resolution: 2mmx1.6mm/0.94mmx0.94mm, slice thickness=8mm, FOV: 300x300x8mm3, TE/TR: 1.47/2.95 ms, α: 45°, temporal resolution of ~50ms (20 cardiac frames).Manual segmentations of the aorta in the 3D CINE scans of 10 volunteers in two cardiac phases, end-systole and mid-diastole, allowed for the training of nnU-Net3. This U-Net automatically segmented all cardiac phases of the 3D CINE scans and its performance was subsequently tested in the other 4 volunteers.
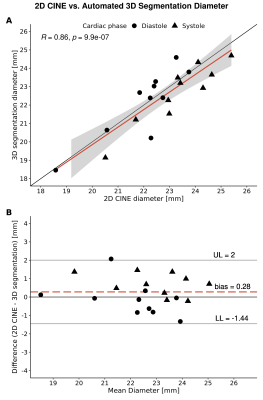
The quality of the segmentations was investigated in a subset of 10 volunteers by comparing diameter measurements on the 2D CINE slice and the corresponding location on the segmentation using Pearson’s correlation and Bland-Altman analysis.
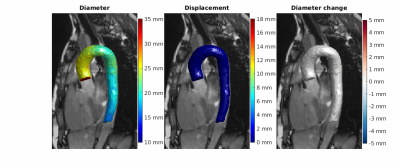
The time-resolved segmentations were used to derive 4D diameter4, and displacement and diameter change (in mm) of the ascending aorta (AAo) were derived using an iterative closest point (ICP) registration method5 of a single reference mid-diastolic phase to all timeframes. The midpoint of the aortic arch was determined on this reference phase and determined the geometric boundary coordinates for the AAo region on that and all other phases. Maximum variability between test-retest-rescan per volunteer was measured as the range (dispmax - dispmin). All continuous variables except Dice score are expressed as median (IQR).
Results
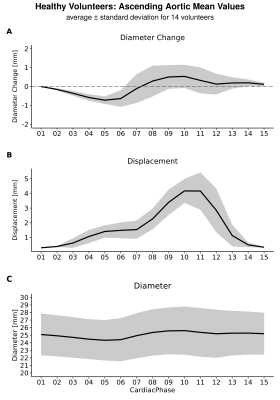
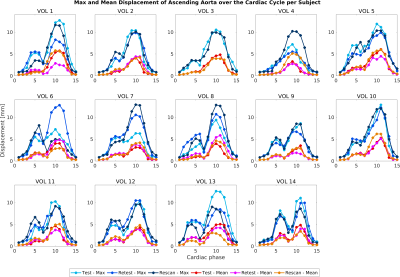
Automated compared to manual segmentation of the test set showed a Dice score of 0.931±0.017. Figure 2 shows the segmentations for each cardiac frame with corresponding diameter, displacement and diameter change for an example volunteer. Figure 3 demonstrates the mean AAo diameter, displacement and diameter change curves averaged (±standard deviation) over all subjects for the test scan. Figure 4 shows the test-retest-rescan comparisons of maximum and mean AAo displacement over the cardiac cycle for all volunteers. Across all volunteers, the systolic maximum and mean AAo displacement of the first scan was 10.3(3.6) mm and 4.2(0.9) mm. The maximum individual variability over the three scans of maximum and mean AAo displacement was respectively 4.2(3.6) mm and 2.2(1.3) mm. Figure 5 shows the agreement between diameter measurements from the 2D CINE and 3D segmentations. Across all volunteers, the mean systolic/diastolic diameter for 2D CINE was 23.4(1.3)/22.3(0.7) mm and for 3D segmentations 23.1(1.9)/22.5(2.1). Pearson’s R between 2D and 3D segmentation was 0.86 and mean difference was 0.28 mm with LOA of ±1.72 mm.Discussion
The 3D motion maps show bulk motion that aligns with the 3D CINE and visible diameter change implying our method captures these adequately, which is also supported by the average curves in figure 3. The variability over test/retest/rescan of maximum AAo displacement was largest in systole, with the majority of volunteers showing reproducible curves. Further development of MRI acquisition, segmentation and registration methods could improve the reproducibility of this metric.The validation of automated segmentation quality by comparing with 2D CINE diameter measurements shows a mean difference close to 0 mm and LOA around 2 mm. As a single aorta specialist may obtain measurements that differ by 3 mm from identical images6, this indicates good agreement. Systolic diameter measures more often show a positive bias than diastolic measures. The aorta being less orthogonal to the 2D imaging plane in systole as a result of cardiac pull in combination with a slice thickness of 8 mm likely results in a cross-section overestimation.
Conclusion
We have presented a novel method based on 3D CINE CMR combined with machine learning segmentation and registration algorithms to visualize and quantify aortic diameter changes and bulk displacement over the cardiac cycle. In future work we will apply this method to patients with Marfan syndrome to investigate whether this genetic disease causes a decrease in aortic diameter change and bulk motion compared to healthy controls and how displacement and diameter change compare to measures of pulse wave velocity.Acknowledgements
This study is part of the project "Comprehensive assessment of 4D thoracic aorta biomechanics using novel cardiac MRI technology" with project number 18402 of the research programme "Applied and Engineering Sciences", which is partly financed by the Dutch Research Council (NWO).References
1. Beller CJ, Labrosse MR, Thubrikar MJ, et al. Role of Aortic Root Motion in the Pathogenesis of Aortic Dissection. Circulation. 2004;109(6):763-769. doi:10.1161/01.CIR.0000112569.27151.F7
2. Merton R, Schrauben EM, Strijkers GJ, et al. Reproducibility of aortic diameter and displacement derived from free-breathing 3D balanced steady-state free precession CINE images at 3T. ISMRM Proceedings, May 2022; https://archive.ismrm.org/2022/1229.html. Accessed November 07, 2022.
3. Isensee, F, Jaeger PF, Kohl SAA, et al. nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation. Nat Methods 2021;18:203-211. doi:10.1038/s41592-020-01008-z
4. van Ooij P, Collins J, Fedak P, et al. 3D Linear Regression Analysis Reveals Relationships of 4D flow MRI-derived Aortic Dimensions with Age, Gender and Wall Shear Stress in Patients with Aortopathy. Proc Intl Soc Mag Reson 25. 2017. p. 0288.
5. Audenaert EA, van Houcke J, Almeida DF, et al. Cascaded statistical shape model based segmentation of the full lower limb in CT. Computer Methods in Biomechanics and Biomedical Engineering. 2019;22(6):644-657. doi:10.1080/10255842.2019.1577828
6. Elefteriades JA, Mukherjee SK, Mojibian H. Discrepancies in Measurement of the Thoracic Aorta: JACC Review Topic of the Week. J. Am. Col. Card. 2020;76(2):201-217.
Figures