0541
Mesoscale distortion-free in-vivo dMRI at 7T using ROtating-view Motion-robust supEr Resolution EPTI (Romer-EPTI)1Athinoula A. Martinos Center for Biomedical Imaging, MGH, Charlestown, MA, United States, 2Department of Radiology, Harvard Medical School, Charlestown, MA, United States, 3Harvard-MIT Health Sciences and Technology, MIT, Cambridge, MA, United States
Synopsis
Keywords: Data Acquisition, Brain
Pushing the spatial resolution of diffusion MRI to mesoscale is technically challenging due to the low SNR efficiency and severe image artifacts. Last year, we presented a novel ROtating-view Motion-robust supEr Resolution Echo Planar Time-resolved Imaging method (Romer-EPTI), providing significant gains (~3.5×) in SNR efficiency and high-quality images free from distortion with minimized motion artifacts. In this work, we further developed Romer-EPTI at 7T for in-vivo mesoscale dMRI. By exploiting the higher signal of 7T and the short-TE, high-SNR, distortion-free acquisition provided by Romer-EPTI, mesoscale whole-brain dMRI at an unprecedented 485-μm isotropic resolution was acquired.Introduction
Pushing the spatial resolution of diffusion MRI to mesoscopic scale is technically challenging due to the low SNR efficiency and elevated image artifacts (e.g., distortion, motion), but can resolve detailed brain structures in white and gray matters and improve tractography1,2. To improve the SNR efficiency, many acquisition methods have been developed including simultaneous multi-slab imaging3-5, SMS-gSlider1,6, and super-resolution reconstruction7-9. Last year, we presented a novel ROtating-view Motion-robust supEr Resolution Echo Planar Time-resolved Imaging method (Romer-EPTI)10, which provides significant gains (~3.5×) in SNR efficiency and high-quality images free from distortion with minimized motion artifacts. Using Romer-EPTI, we were able to acquire whole-brain diffusion data at 500-μm isotropic resolution at 3T, revealing detailed U-fibers and gray matter structures10. In this work, to further increase the image SNR for in-vivo mesoscale dMRI, we further developed Romer-EPTI at 7T to take the advantage of the higher signal from the ultra-high field11 and the short-TE and distortion-free acquisition provided by Romer-EPTI. In addition, k-t space partial Fourier acquisition12 and spatiotemporal encoding optimization were employed for EPTI to reduce the acquisition shots for shorter volume TR and improved motion robustness. Higher image SNR was observed at 7T over 3T, and mesoscale whole-brain dMRI dataset at an unprecedented 485-μm isotropic resolution was acquired using Romer-EPTI at 7T.Methods
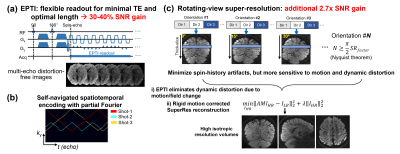
Romer-EPTI: Fig.1 illustrates the Romer-EPTI method. The use of EPTI13,14 acquisition enables flexible readout with its time-resolving approach and allows for minimized TE and optimal readout length, providing 30-40% SNR gain over EPI. Self-navigated spatiotemporal encoding was designed to correct the shot-to-shot phase variation, and k-t partial Fourier12 was used to reduce the acquisition shots (only 3 shots are required for 485μm resolution). Romer-EPTI acquires multiple thick-slice volumes with short volume TR and different slice orientations using a rotating-view super-resolution approach7-10 to resolve the high isotropic resolution volume by encoding and rotating the readout and slice dimensions.To minimize the spin-history artifacts with short TR, it is important to acquire all the diffusion directions of the same orientation first. However, this will lead to long time distance between different slice orientations, making the acquisition more sensitive to motion. In addition to rigid motion, the motion-related dynamic distortion between orientations is challenging to model and correct which will lead to blurring and artifacts. The use of EPTI solves this challenge by eliminating all the dynamic distortion due to motion or field change from the acquisition, and the rigid motion is then corrected in the super-resolution reconstruction (Fig.1c). Here, we acquired N=12 orientations for a super-resolution factor of 8 for mesoscale dMRI, that can offer 2.7× SNR gain. Together, Romer-EPTI can provide 3.5× higher SNR efficiency with minimized artifacts over conventional EPI acquisition.
485μm-isotropic in-vivo dMRI at 7T: 56-direction diffusion data were acquired using Romer-EPTI at 7T Siemens Terra with a 32-channel nova coil. The key imaging parameters are: TE (spin-echo) = 41ms, TR = 3.2s, super-resolution factor = 8, 3-shot EPTI acquisition with partial Fourier of 5/8, multiband factor=2, b-value = 1000s/mm2, scan time ~110 minutes. Real value diffusion15 was used to further reduce the noise in processing.
Results
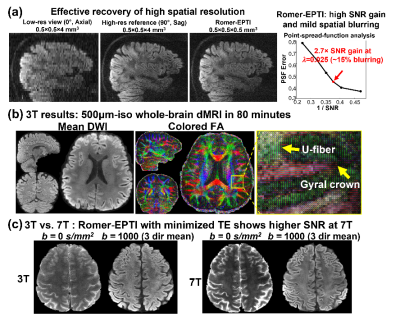
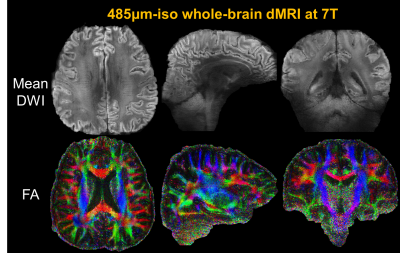
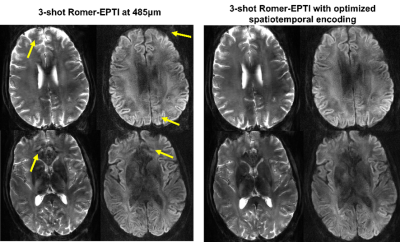
Fig.2a shows the effectiveness of the spatial resolution recovery of Romer-EPTI. The PSFerror vs. 1/SNR L-curve analysis shows that using a λ of 0.025 in the super-resolution reconstruction, Romer provides a SNR gain of 2.7× with moderate blurring (~15% blurring). Fig.2b shows the 500μm-iso dataset acquired 3T in 80 minutes. Although the SNR is still limited at 3T, high-quality DWI and tensors were obtained and reveal clear U-fibers and fibers in the gyral crowns and walls. The SNR gain of 7T can be compromised when using long TE due to the shortened T2 values. The developed short-TE EPTI acquisition reduces this effect and provides higher SNR images at 7T over 3T shown in Fig.2c. Using Romer-EPTI, mesoscale in-vivo dMRI data were acquired at 7T at an unprecedented 485μm-isotropic resolution shown in Fig.3. The mean DWI and FA maps show good image SNR and resolution, however, darkness in the central and lateral-bottom areas of the brain was observed due to the much severe B1 inhomogeneity at 7T. In addition, artifacts are observed at 7T as shown in Fig.4 around regions with large B0 inhomogeneity (e.g., frontal lobe and regions near nasal cavity). By optimizing the spatiotemporal encoding of EPTI with a larger acceleration along phase-encoding and therefore closer temporal sampling, such artifacts were substantially reduced, and cleaner high-resolution images were obtained.Conclusion
Romer-EPTI achieves high SNR distortion-free imaging with minimized motion and spin-history artifacts for mesoscale dMRI. Compared with 3T, 7T provides higher SNR for ultra-high resolution dMRI but also faces challenges including B1 inhomogeneity, and next we will integrate parallel transmission to improve the B1 field homogeneity and employ the optimized EPTI encoding to further improve the data quality and analyze the detailed structures in cortical gray matter.Acknowledgements
This work was supported by the NIH NIBIB (R01-EB019437, P41EB030006) and the instrumentation Grants (S10OD023637, S10-RR023401, S10-RR023043, and S10-RR019307).References
1 Setsompop, K. et al. High-resolution in vivo diffusion imaging of the human brain with generalized slice dithered enhanced resolution: Simultaneous multislice (gSlider-SMS). Magnetic resonance in medicine 79, 141-151, doi:10.1002/mrm.26653 (2018).
2 Schilling, K. et al. Confirmation of a gyral bias in diffusion MRI fiber tractography. Human brain mapping 39, 1449-1466, doi:10.1002/hbm.23936 (2018).
3 Frost, R., Miller, K. L., Tijssen, R. H., Porter, D. A. & Jezzard, P. 3D multi-slab diffusion-weighted readout-segmented EPI with real-time cardiac-reordered K-space acquisition. Magnetic resonance in medicine 72, 1565-1579, doi:10.1002/mrm.25062 (2014).
4 Holtrop, J. L. & Sutton, B. P. High spatial resolution diffusion weighted imaging on clinical 3 T MRI scanners using multislab spiral acquisitions. J Med Imaging (Bellingham) 3, 023501, doi:10.1117/1.JMI.3.2.023501 (2016).
5 Engstrom, M. & Skare, S. Diffusion-weighted 3D multislab echo planar imaging for high signal-to-noise ratio efficiency and isotropic image resolution. Magnetic resonance in medicine 70, 1507-1514, doi:10.1002/mrm.24594 (2013).
6 Wang, F. et al. Motion-robust sub-millimeter isotropic diffusion imaging through motion corrected generalized slice dithered enhanced resolution (MC-gSlider) acquisition. Magnetic resonance in medicine doi:10.1002/mrm.27196 (2018).
7 Plenge, E. et al. Super-resolution methods in MRI: can they improve the trade-off between resolution, signal-to-noise ratio, and acquisition time? Magnetic resonance in medicine 68, 1983-1993, doi:10.1002/mrm.24187 (2012).
8 Shilling, R. Z. et al. A super-resolution framework for 3-D high-resolution and high-contrast imaging using 2-D multislice MRI. IEEE Trans Med Imaging 28, 633-644, doi:10.1109/TMI.2008.2007348 (2009).
9 Vis, G., Nilsson, M., Westin, C. F. & Szczepankiewicz, F. Accuracy and precision in super-resolution MRI: Enabling spherical tensor diffusion encoding at ultra-high b-values and high resolution. NeuroImage 245, 118673, doi:10.1016/j.neuroimage.2021.118673 (2021).
10 Dong, Z., Polimeni, J. R., Wald, L. L. & Wang, F. SuperRes-EPTI: in-vivo mesoscale distortion-free dMRI at 500μm-isotropic resolution usingshort-TE EPTI with rotating-view super resolution In Proceedings of the 30th Annual Meeting of ISMRM 2022.
11 Wu, W. et al. High-resolution diffusion MRI at 7T using a three-dimensional multi-slab acquisition. Neuroimage 143, 1-14 (2016).
12 Wang, F., Dong, Z., Setsompop, K., Polimeni, J. R. & Wald, L. L. Improving fMRI acquisition using single-shot EPTI with distortion-free high-SNR high-CNR multi-echo imaging In Proceedings of the 30th Annual Meeting of ISMRM 2022.
13 Dong, Z., Wang, F., Wald, L. & Setsompop, K. SNR‐efficient distortion‐free diffusion relaxometry imaging using accelerated echo‐train shifted echo‐planar time‐resolving imaging (ACE‐EPTI). Magnetic Resonance in Medicine 88, 164-179 (2022).
14 Wang, F. et al. Echo planar time-resolved imaging (EPTI). Magnetic resonance in medicine 81, 3599-3615, doi:10.1002/mrm.27673 (2019).
15 Eichner, C. et al. Real diffusion-weighted MRI enabling true signal averaging and increased diffusion contrast. NeuroImage 122, 373-384, doi:10.1016/j.neuroimage.2015.07.074 (2015).
Figures