0539
Rapid Mesoscale MP2RAGE Imaging at Ultra High Field with Controlled Aliasing1Centre for Functional and Metabolic Mapping (CFMM), Robarts Research Institute, Western University, London, ON, Canada, 2Department of Medical Biophysics, Schulich School of Medicine and Dentistry, Western University, London, ON, Canada, 3Siemens Medical Solutions USA, Boston, MA, United States, 4Siemens Healthcare Limited, Oakville, ON, Canada, 5Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital & Harvard Medical School, Boston, MA, United States, 6Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Boston, MA, United States, 7Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Boston, Boston, MA, United States, 8Fetal-Neonatal Neuroimaging & Developmental Science Center, Boston Children’s Hospital, Boston, MA, United States, 9Department of Radiology, Harvard Medical School, Boston, MA, United States
Synopsis
Keywords: Data Acquisition, New Trajectories & Spatial Encoding Methods
MP2RAGE is considered the workhorse sequence at ultra-high field (UHF) as it provides high-contrast and bias-free structural imaging for both anatomical and segmentation purposes. However, high-resolution MP2RAGE imaging is hampered by lengthy acquisitions, which can be partially mitigated using parallel imaging, but this comes at the cost of g-factor and √R penalties on SNR; thus, limiting the usefulness of this sequence at the submillimeter scale. In this work, we show preliminary results on how wave encoding, and blipped-controlled aliasing could allow efficient MP2RAGE acquisitions at up to 560 um isotropic resolution at 7T.Introduction
MP2RAGE1 is considered the workhorse sequence at 7T as it provides high-contrast and bias-free structural imaging for both anatomical and segmentation purposes. While this nearly doubles the acquisition time compared to standard MPRAGE, MP2RAGE mitigates the bias field and B1+ effects and contributions from T2* and proton density weighting to provide high-quality T1-weighted images. While the inversion pulse provides the desired T1 contrast, it severely impacts the SNR. Moreover, MP2RAGE becomes a prohibitively long acquisition for mesoscale resolutions. Therefore, the acquisition and reconstruction pipeline should compensate for such contrast and image encoding challenges of submillimeter imaging while enabling feasible acquisition times. From the acquisition-side, the incorporation of blipped-controlled aliasing (blipped-CAIPI)2 and wave encoding3 improves g-factor4 penalty for highly accelerated acquisitions, thus addressing the image encoding challenge. Going to ultra- high fields and using massively parallel receive arrays provide additional sensitivity, thus mitigating the SNR barriers due to contrast encoding. Hence, in this work we present preliminary results to render the MP2RAGE sequence faster and more robust to SNR reduction. Finally, we reach a 560um isotropic resolution MP2RAGE acquisition in 8:43 by capitalizing on a custom 64-chan head array on a 7T platform.Methods
This study was approved by Institutional Review Boards and informed consent was obtained prior to scanning. Elliptical k-space sampling was performed for additional speed-up in all acquisitions. Adiabatic inversion pulses were used to combat field inhomogeneities during inversion.Siemens MAGNETOM 7T Plus - Acquisition and Offline Reconstruction (at London, Canada)
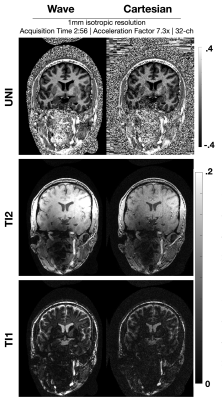
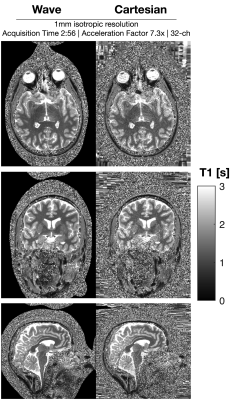
Acquisition was performed using a 32-channel receive and 8-channel transmit head-coil. Cartesian and Wave data at 1 mm isotropic resolutions were acquired with acceleration factor of 3x2 in PE/PAR directions. Both sequences have a total acquisition time of 2:56. Additional sequence parameters: FOV = 240x240x192 mm; TE-Cartesian(TE-Wave)/TI1/TI2/TR = 3.18(3.27)/900/2500/5000 ms; FA1/FA2 = 4/5; echo spacing = 7.7 (Cartesian)/7.9 (Wave) ms; Bandwidth = 200 Hz/Px. Wave parameters were Gmax 40 mT/m; 14 Cycles; Gslew-rate 180.0 mT/m/ms. Datasets underwent through the following reconstruction pipeline: the reference scan was used for estimation of sensitivity coil maps using ESPIRIT5 (Bart toolbox v0.8.00) after coil-compression6 to 21 virtual coils. The point-spread-function from Wave encoding was estimated using autoPSF7, and the actual reconstruction was performed using Wave-CAIPI3.
Siemens Terra 7T - Acquisition and Online Reconstruction (at Boston, USA)
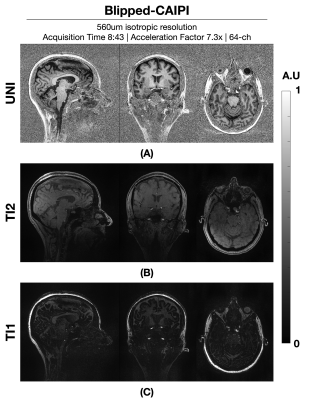
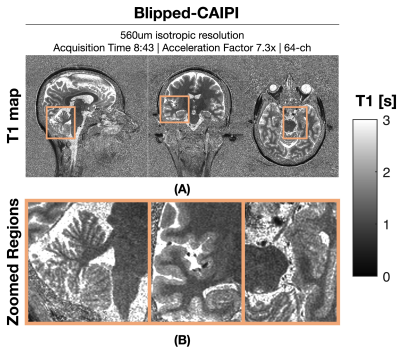
Acquisition was performed using a custom made 63-channel receive8 and 1-channel transmit receive. A Cartesian 560um isotropic resolution with acceleration factor of 3x2 in PE/PAR directions; total acquisition time: 8:43. Additional sequence parameters: FOV = 240x240x192 mm; TE/TI1/TI2/TR = 3.46/900/2500/5000 ms; FA1/FA2 = 4/5; echo spacing = 8.6 ms; Bandwidth = 200 Hz/Px.
Uniform (UNI) Images and T1 maps
UNI images were computed according to1 which helped mitigate biases due to B1- and B1+ inhomogeneity. We used a dedicated MP2RAGE toolbox (https://github.com/JosePMarques/MP2RAGE-related-scripts) to estimate T1 maps1.
Results
Figure 1: shows a comparison between wave and Cartesian encoded data at R=7.3-fold net acceleration at 1 mm resolution using 32ch reception. Wave-MP2RAGE largely mitigated the noise amplification present in the Cartesian encoded data and led to high quality UNI images from a 2:56 min acquisition. Figure 2: depicts the corresponding T1 maps obtained from the wave and Cartesian data presented in Figure 1, where the gain in g-factor noise mitigation is similarly observed. Figure 3: UNI as well as the individual TI images from the 560um isotropic blipped-CAIPI acquisition at R=7.3-fold acquisition are shown. The custom 64ch head coil provided drastic gains in sensitivity and parallel imaging capability to yield high quality data. Figure 4: depicts the corresponding T1 maps obtained from figure 3. Insets zoom into the cerebellum, medial temporal lobe, and the vicinity of the brain stem to demonstrate the signal uniformity and detail.Discussion and Conclusion
In this work we have shown preliminary results on a new MP2RAGE sequence at 7T that enables both blipped-controlled aliasing and wave encoded acquisition strategies. Combined with massively parallel receive arrays, our acquisitions allowed us to surpass the limits from conventional Cartesian MP2RAGE, and to reach a 560um isotropic resolution MP2RAGE acquisition in 8:43.Acknowledgements
This work was funded by a CIHR Foundation Grant to RM and an NSERC Discovery Grant.This work was funded by NIH grant numbers R01EB028797, R01EB032378, U01EB025162, P41EB030006, U01EB026996, R03EB031175, and NVIDIA gpu.We thank ISMRM for the Exchange research program award.References
1. Marques JP, Kober T, Krueger G, Zwaag W van der, Moortele PFV de, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage. 2010;49(2):1271-1281. doi:10.1016/j.neuroimage.2009.10.002
2. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped‐controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g‐factor penalty. Magnet Reson Med. 2012;67(5):1210-1224. doi:10.1002/mrm.23097
3. Bilgic B, Gagoski BA, Cauley SF, et al. Wave‐CAIPI for highly accelerated 3D imaging. Magnet Reson Med. 2015;73(6):2152-2162. doi:10.1002/mrm.25347
4. Larkman DJ. Parallel Imaging in Clinical MR Applications. Medical Radiology. 2007:37-48. doi:10.1007/978-3-540-68879-2_3
5. Uecker M, Lai P, Murphy MJ, et al. ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magnet Reson Med. 2014;71(3):990-1001. doi:10.1002/mrm.24751
6. Buehrer M, Pruessmann KP, Boesiger P, Kozerke S. Array compression for MRI with large coil arrays. Magnet Reson Med. 2007;57(6):1131-1139. doi:10.1002/mrm.21237
7. Cauley SF, Setsompop K, Bilgic B, Bhat H, Gagoski B, Wald LL. Autocalibrated wave‐CAIPI reconstruction; Joint optimization of k‐space trajectory and parallel imaging reconstruction. Magnet Reson Med. 2017;78(3):1093-1099. doi:10.1002/mrm.26499
8. Mareyam A, Kirsch JE, Chang Y, Madan G, Wald LL. A 64-Channel 7T array coil for accelerated brain MRI. In: Proceedings of the 28th Annual Meeting of ISMRM, Virtual.; 2020:764.