0536
Variable-flip-angle 3D spiral-in-out TSE/SPACE using echo-reordering and concomitant gradient compensation at 0.55 T1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Cardiovascular Branch, Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 3Radiology & Medical Imaging, University of Virginia, Charlottesville, VA, United States
Synopsis
Keywords: Data Acquisition, Data Acquisition
This study describes an alternative approach to Cartesian SPACE for 1 mm3 isotropic whole brain T2-weighted imaging on a high performance 0.55T scanner. In this technique, the Cartesian readouts were replaced by interleaved, rotated spiral-in-out trajectories, combined with a variable-flip-angle refocusing, echo-reordering, and concomitant gradient compensation. Parallel imaging (PI) and compressed sensing (CS) were utilized for further acceleration. Compared to 3D-Cartesian SPACE, this method can be leveraged to mitigate the lower SNR of 0.55T via the improved SNR efficiency of prolonged spiral trajectory sampling.Introduction:
Cartesian SPACE uses very long echo trains to increase scan efficiency by varying the flip-angles of the refocusing RF pulses.1,2 However, 3D-Cartesian sampling remains time-consuming because of its relatively inefficient k-space coverage when prescribing high-isotropic spatial resolution. This problem worsens at low-fields due to the inherently lower SNR, requiring several signal averages to maintain clinically acceptable image quality.3Spiral acquisitions cover k-space more efficiently than Cartesian, providing a means to reduce scan time or improve SNR.4,5 Spiral acquisitions are attractive at low fields, where improved absolute field homogeneity enables imaging with prolonged readouts for higher SNR.6 However, concomitant (Maxwell) gradients at low fields may disrupt signal pathways in TSE imaging, especially when using high amplitude partition encodings and/or time-varying spiral readouts, wherein phase errors induced by concomitant gradients vary along the echo train.7-9
In this work, we develop a 3D-TSE method using interleaved, rotated spiral-in-out readouts, incorporating a variable-flip-angle approach with echo-reordering to shape the signal evolution, and parallel imaging/compressed sensing to further accelerate data acquisition at 0.55T. Additionally, we include sequence modifications and an image reconstruction method to compensate for concomitant gradient effects.
Methods:
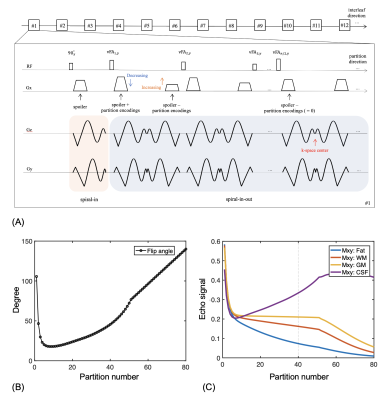
A simplified timing diagram of the method for a sagittal slice orientation is shown in Figure 1A. Spiral interleaves were rotated in the outer loop, where each excitation acquires a single spiral-in-out arm for every 3D-partition encoding, while the inner loop incremented the partition encodings along the echo direction.To improve scan efficiency (RO: 4.48 ms, ESP: 7.2 ms), sequence modifications included:
- Non-spatially-selective refocusing RF pulses.
- Spoiler gradients (~4π dephasing) combined with partition-encodings.
- Simulataneous spiral-in-out prewinder/rewinder lobes with spoiler/partition-encoding gradients.
- Constant-density and variable-density spirals used for fully-sampled, and 2X-accelerated undersampled data, respectively.
Encoding gradients with variable amplitudes along the echo train will produce unbalanced concomitant fields that need to be corrected via sequence modifications.8,9 To reduce self-squared Maxwell terms:
- Gradient de-rating used for partition-encodings.
- Spiral-in gradients (the first half of the following spiral-in-out readout) added in the interval between the excitation pulse and the first refocusing pulse.
The variable-flip-angle RF series shown in Figure 1B was calculated for gray matter specifically at 0.55T (T1 800 ms, T2 110 ms) using the EPG algorithm.2 For image reconstruction, residual phase errors from concomitant gradients during corrected by a fast conjugate phase reconstruction method based on Chebyshev approximation. Trajectory correction was performed using the GIRF method10, and echo-reordering was applied whereby the echo order was reversed during the second average11. L1-ESPIRiT12 was utilized for undersampled datasets.
Experiments were performed on a ramped-down 0.55-T MR scanner (prototype MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany) using a 16-channel head/neck coil. For both phantom and healthy volunteer studies (n = 3), images were acquired using spiral SPACE with and without compensation, and Cartesian SPACE as a reference.
Results and Discussion:
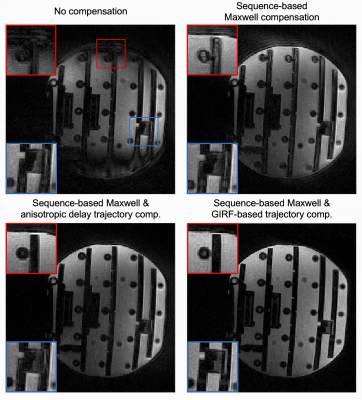
Figure 1C shows the signal evolutions for white matter, gray matter, fat, and cerebral spinal fluid. Note that fat signal is much lower than the others, so fat saturation was not needed.Figure 2 illustrates the performance of the proposed sequence-based Maxwell compensation and trajectory correction in a phantom. Signal loss is seen at the top and bottom of Figure 2A because of strong concomitant fields along the physical Z axis. After Maxwell compensation, Figure 2B shows much improved image quality but still demonstrates edge artifacts and signal shading due to trajectory infidelity. Applying trajectory correction using the GIRF method (Figure 2D) yields better image quality than an anisotropic gradient delay model (Figure 2C).
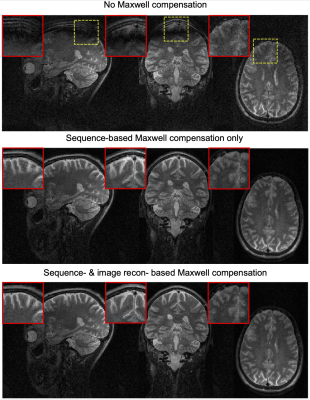
Figure 3 shows brain images from spiral SPACE with no concomitant field compensation (top), with sequence-based concomitant field compensation (middle), or with full concomitant field compensation (sequence and reconstruction, bottom). Signal loss and blurring artifacts are substantially reduced when using sequence-based concomitant field compensation (zoomed in regions), and residual blurring artifacts accrued during readout are further mitigated through image reconstruction.
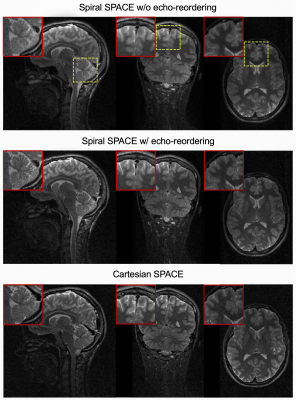
Figure 4 shows fully-corrected spiral SPACE images without echo-reordering (top) and with echo-reordering (middle), compared to Cartesian SPACE images (bottom). Data after echo-reordering and averaging was further normalized based on a simulated signal decay from EPG, and corresponding images show improved sharpness compared to those without echo-reordering11.
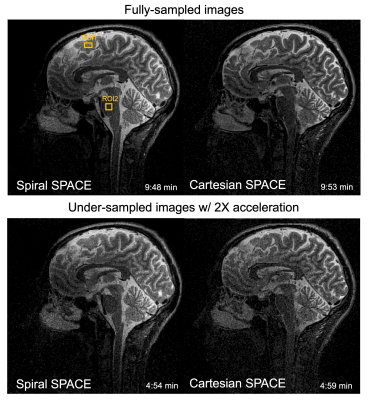
Figure 5 displays images reconstructed with fully-sampled (top, ~10 mins.) and 2X-undersampled (bottom, ~5 mins.) acquisitions by spiral SPACE (left) and Cartesian SPACE (right). SNR measurements using ROIs were performed on fully-sampled images only, and showed increased apparent SNR values when using spiral SPACE over Cartesian SPACE (17±12.3% gain) for similar scan times.
Conclusion:
We demonstrated a 3D spiral SPACE pulse sequence that incorporates variable flip angles with an echo-reordering strategy, concomitant gradient compensation, and variable-density undersampling for fast T2-weighted brain imaging at 0.55T. This approach is an attractive alternative to the conventional Cartesian SPACE for brain images with improved SNR.Acknowledgements
The authors would like to acknowledge the assistance of Siemens Healthcare in the modification of the MRI system for operation at 0.55 T under an existing cooperative research agreement (CRADA) between NHLBI and Siemens Healthcare. This work was supported in part by Siemens Healthcare.References
[1] Mugler JP 3rd. Optimized three-dimensional fast-spin-echo MRI. J Magn Reson Imaging. 2014;39(4):745-767. doi:10.1002/jmri.24542
[2] Hennig J, Weigel M, Scheffler K. Calculation of flip angles for echo trains with predefined amplitudes with the extended phase graph (EPG)-algorithm: principles and applications to hyperecho and TRAPS sequences. Magn Reson Med 2004; 51: 68– 80.
[3] Campbell-Washburn AE et al. Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology 2019; 293:384-393.
[4] Li Z, Wang D, Robison RK, et al. Sliding-slab three-dimensional TSE imaging with a spiral-in/out readout. Magn Reson Med. 2016;75:729-738.
[5] Wang Z, Allen SP, Feng X, Mugler JP, Meyer CH. SPRING-RIO TSE: 2D T2-Weighted Turbo Spin-Echo Brain Imaging using SPiral RINGs with Retraced In/Out Trajectories. Magn Reson Med. 2022;88:601-616.
[6] Restivo MC, Ramasawmy R, Bandettini WP, Herzka DA, Campbell-Washburn AE. Efficient spiral in-out and EPI balanced steady-state free precession cine imaging using a high-performance 0.55T MRI. Magn Reson Med. 2020;84:2364–2375.
[7] Zhou XJ, Tan SG, Bernstein MA. Artifacts induced by concomitant magnetic field in fast spin-echo imaging. Magn Reson Med 1998; 40:582-591.
[8] Mugler JP, Campbell-Washburn AE, Ramasawmy R, Pfeuffer J, Meyer, CH. Maxwell Compensation for Spiral Turbo-Spin-Echo Imaging. Proceedings 29th Annual Meeting ISMRM, Virtual meeting. 2021:0003.
[9] Wang Z, Feng X, Ramasawmy R, Campbell-Washburn AE, Mugler JP, Meyer CH. Maxwell Field Compensation for 2D Spiral-Ring Turbo Spin-Echo Imaging at 0.55T and 1.5T. In Proceedings of the 31st Annual Meeting of ISMRM, London, UK, 2022:0319.
[10] Vannesjo SJ, Haeberlin M, Kasper L, Pavan M, Wilm BJ, Barmet C, Pruessmann KP. Gradient system characterization by impulse response measurements with a dynamic field camera. Magn Reson Med 2013; 69: 583–593.
[11] Li Z, Karis JP, Pipe JG. A 2D spiral turbo-spin-echo technique. Magn Reson Med. 2018;80:1989–1996.
[12] Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, Lustig M. ESPIRiT–an eigenvalue approach to auto-calibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med. 2014;71:990–1001.
Figures