0528
VISIBLE: Improvement in Vessel Visibility and Application of Machine Learning to Detect Brain Metastases
Kazufumi Kikuchi1, Osamu Togao2, Koji Yamashita3, Makoto Obara4, and Kousei Ishigami1
1Department of Clinical Radiology, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan, 2Department of Molecular Imaging & Diagnosis, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan, 3Department of Radiology Informatics & Network, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan, 4Philips Japan, Tokyo, Japan
1Department of Clinical Radiology, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan, 2Department of Molecular Imaging & Diagnosis, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan, 3Department of Radiology Informatics & Network, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan, 4Philips Japan, Tokyo, Japan
Synopsis
Keywords: Tumors, Machine Learning/Artificial Intelligence, Brain metastases
This study aimed to improve vessel visibility by modifying k-space filling and to verify the usefulness of volume isotropic simultaneous interleaved bright- and black-blood examination (VISIBLE) in detecting brain metastases using machine learning (ML). We tested three types of VISIBLE in different k-space fillings, and counted the number of vessels. We also tested the ML model by using VISIBLE. The number of vessels was lower in Centric and Reversed centric sequences than that in MPRAGE, but comparable in the Startup echo 30 sequence. Our ML model was achieved high sensitivity (97%) and there were no differences among three sequences.INTRODUCTION
Volume isotropic simultaneous interleaved bright- and black-blood examination (VISIBLE) allows for simultaneous acquisition of images with (Black) and without (Bright) blood vessel suppression1,2. VISIBLE can detect small brain metastases compared with the conventional contrast-enhanced 3D MR sequence, magnetization-prepared rapid acquisition of gradient echo (MPRAGE). However, vessel visibility on Bright images during VISIBLE is lesser than that on MPRAGE2. First, we aimed to improve the vessel visibility of Bright by modifying k-space filling. Second, we aimed to verify the usefulness of VISIBLE for detecting brain metastases using machine learning (ML).METHODS
[VISIBLE Sequence] The technical details of VISIBLE have been reported previously1. Briefly, the VISIBLE sequence is based on a 3D T1-turbo field-echo (TFE) sequence. To suppress blood signals, this sequence has a black-blood prepulse called motion-sensitized driven equilibrium (MSDE). After MSDE preparation, two sequential phases of TFE were implemented: T1-TFE with MSDE, providing Black images, and T1-TFE without MSDE, providing Bright images. We tested three types of VISIBLE sequences to modify the k-space filling (Fig. 1). The Centric sequence is a prototype. The Reversed centric sequence fills the k-space in a Reversed centric order in the Bright image to improve vessel visibility. The Startup echo 30 sequence implements dummy echoes before the Bright image to further improve vessel visibility.[Vessel Counting] The number of visualized blood vessels was counted in a single semi-oval centrum of the brain on the three types of VISIBLE images and compared to that counted on MPRAGE in 40 patients without metastases. The first post-contrast scan was initiated 5 min after contrast injection. To avoid timing biases, we alternated the order of the two post-contrast sequences after the injection as follows: order 1, Start echo 30 → Reversed centric → Centric → MPRAGE; and order 2, Centric → Reversed centric → Startup echo 30 → MPRAGE. Statistical comparisons were performed using one-way ANOVA followed by Dunnett's multiple-comparison test.
[AI-VISIBLE Analysis] The details of the ML model for VISIBLE in detecting brain metastases have been reported previously3. Briefly, the training data (50 patients/165 lesions) were used to establish the ML model. The sensitivity and false-positives (FPs) in detecting brain metastases were evaluated among these three VISIBLE sequences using the ML model in 30 patients. Statistical comparisons were performed using one-way ANOVA, followed by the Friedman test and Dunn's multiple comparisons test.
RESULTS
The number of vessels overall was significantly less in the Centric (39.3 ± 9.7, P < 0.0001) and Reversed centric (44.2 ± 9.8, P = 0.0488) sequences compared to MPRAGE (49.3 ± 9.1) but comparable in the Startup echo 30 (48.1 ± 9.9) sequence (Fig. 2). Figure 3 shows a representative case of metastasis imaged with the three types of VISIBLE sequences and MPRAGE. There were no significant differences in sensitivity among the three sequences (97% in each). However, significantly fewer FPs were achieved with Reversed centric (54 FPs/30 cases) compared to those achieved with Centric (85 FPs) and Startup echo 30 (68 FPs) sequences (P = 0.0092) (Fig. 4). Figure 5 shows a representative case with a metastasis imaged with the three types of VISIBLE using the ML.DISCUSSION
This study demonstrated that the vessel visibility on Bright images can be significantly influenced by the k-space acquisition strategy. If the center of the k-space is acquired too early, the vessel signal will recover from MSDE suppression, which may reduce number of enhanced vessels4. Thus, the Centric sequence showed significantly fewer visualized vessels. Furthermore, insufficiently recovered blood vessels can closely mimic lesions, resulting in an increased FP rate5. In the evaluated setup, the Reversed centric filling, as well as the Startup echo scan, were preferable to achieve improved vessel visibility. In this study, our ML model of VISIBLE achieved high sensitivity with a low FP rate, indicating that our ML model may help radiologists reduce oversight of brain metastases. Sensitivity did not change in the three types of sequences; however, the number of FP achieved was the lowest in Reversed centric. FPs in our ML model mainly resulted in three findings: vessels, noises/artifacts, and the choroid plexus3. Radiologists can easily recognize these findings and so we believe that the FPs on the ML model are trivial and insignificant.CONCLUSIONS
Vessel visibility of the Bright images during VISIBLE were improved by modifying the sequence parameters. In addition, VISIBLE was a useful tool for detecting brain metastases when used in conjunction with ML.Acknowledgements
No acknowledgement found.References
- Yoneyama M, Obara M, Takahara T, et al. Volume isotropic simultaneous interleaved black- and bright-blood imaging: a novel sequence for contrast-enhanced screening of brain metastasis. Magn Reson Med Sci. 2014;13(4):277-84.
- Kikuchi K, Hiwatashi A, Togao O, et al. 3D MR sequence capable of simultaneous image acquisitions with and without blood vessel suppression: utility in diagnosing brain metastases. Eur Radiol. 2015 Apr;25(4):901-10.
- Kikuchi Y, Togao O, Kikuchi K, et al. A deep convolutional neural network-based automatic detection of brain metastases with and without blood vessel suppression. Eur Radiol. 2022 May;32(5):2998-3005.
- Seng K, Maderwald S, de Greiff A, et al. Dynamic contrast-enhanced magnetic resonance angiography of the thoracic vessels: an intraindividual comparison of different k-space acquisition strategies. Invest Radiol. 2010 Nov;45(11):708-14.
- Nagao E, Yoshiura T, Hiwatashi A, et al. 3D turbo spin-echo sequence with motion-sensitized driven-equilibrium preparation for detection of brain metastases on 3T MR imaging. AJNR Am J Neuroradiol. 2011 Apr;32(4):664-70.
Figures
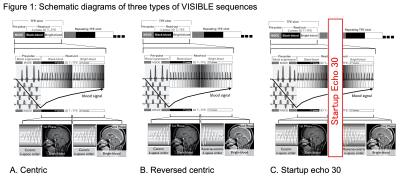
A. The Centric sequence first fills the center of the k-space in both the Black and Bright images.
B. The Reversed centric sequence first fills the center of the k-space in the Black image, followed by filling reverse-centric in the Bright image to improve the vessel visibility.
C. The Startup echo 30 sequence is almost the same as Reversed centric except for adding a dummy echo before Bright image acquisition.
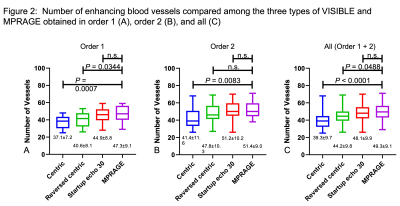
The number of vessels in
all (C) was significantly fewer in Centric and Reversed centric sequences
compared to those in MPRAGE but comparable in Startup echo 30 sequence.
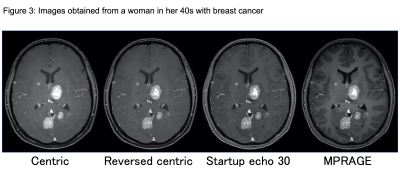
The visibility of vessels increases in the order of
Centric, Reversed centric, and Startup
echo 30 sequences.
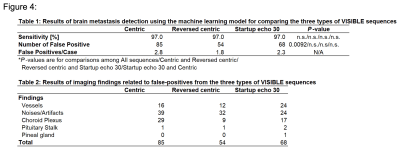
Table 1. Results of brain metastasis detection using the machine learning model for comparing the three types of VISIBLE sequences
Table 2. Results of imaging findings related to false-positives from the three types of VISIBLE sequences
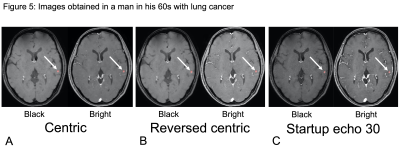
Our machine learning model can detect small
brain metastases in the left temporal lobe. The visibility of vessels in the bright
image improves in the order of Centric, Reversed Centric, and Startup Echo
30 sequences.
DOI: https://doi.org/10.58530/2023/0528