0515
Magnetic resonance elastography-derived stiffness: potential biomarker for differentiation of benign and malignant pancreatic masses1Shanghai Institute of Medical Imaging, Shanghai, China, 2MR Collaboration, Central Research Institute, United Imaging Healthcare, Shanghai, China
Synopsis
Keywords: Pancreas, Cancer
Magnetic resonance elastography (MRE) is a noninvasive technique capable of quantifying tissue mechanical properties (stiffness) in vivo. Given its specialty, it is supposed to be potential for identifying malignant tumors that are characterized by a marked desmoplastic reaction and build-up of fibrotic tissues in cancer cells and tumor microenvironment. However, the field has been largely restricted to studies in pancreatic MRE. This study sought to determine the diagnostic performance of MRE for pancreatic solid masses, compared with diffusion-weighted imaging (DWI) and serum CA19-9, to establish a threshold for differentiating between pancreatic ductal adenocarcinoma (PDAC) and benign tumors in pancreas.
Introduction
Pancreatic ductal adenocarcinoma (PDAC), one of the most lethal diseases, remains a highly fatal malignancy whose 5-year survival rate is only 10%. It accounts for approximately 85–90% of all malignant pancreatic tumors.1 To diagnose inchoate PDAC, many radiological methodologies have applied. MRE is a relatively advanced technique of MRI that can evaluate tissue mechanical properties noninvasively and assess tissue fibrosis in vivo.2–4 Consider of PDAC is a hard mass with a significant desmoplastic reaction and rich fibrotic tissues, while benign pancreatic masses tend to have lower mean collagen content in histology. It suggests that the stiffness of PDAC should be higher than that of other benign masses. Thus, in this prospective study, we aimed to explore the diagnostic value of using MRE for differentiation with PDAC and other benign pancreatic masses preoperatively, and compare it with that of using DWI and serum CA19-9 concentration.Methods
45 patients with pathologically or clinically confirmed pancreatic solid tumor, conducted both MRE and DWI examinations were included in this prospective study. All subjects underwent both MRE and DWI examinations using a 3.0 T MR scanner (uMR 790, United Imaging Healthcare Co Ltd, Shanghai, China). For MRE protocol, the sequence was included using a passive driver placed centered on the xiphisternum to deliver 60-Hz pneumatic vibrations generated by an active acoustic actuator. The two-dimensional spin-echo echoplanar imaging (SE-EPI) sequence was used to obtain four axial stiffness maps of the liver using the following parameters: TR/TE/FA: 3800 ms/86.2 ms/90°; Matrix/FOV: 300×380/300×380 mm2. Wave images depicting shear waves in the pancreas were processed on the scanner using direct inversion algorithm to generate elastograms depicting stiffness in units of kilopascals (kPa). Then tumor stiffness (kPa), pancreatic parenchyma stiffness (kPa) and stiffness ratio were obtained with recourse to elastogram by software 3D slicer (version 4.8.1). The values of all parameters were compared between the subgroups by using the Student t test or Mann-Whitney U test appropriately. Receiver operating characteristic analysis was performed to evaluate the diagnostic performance.Results
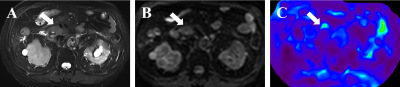
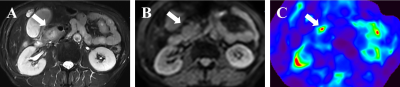
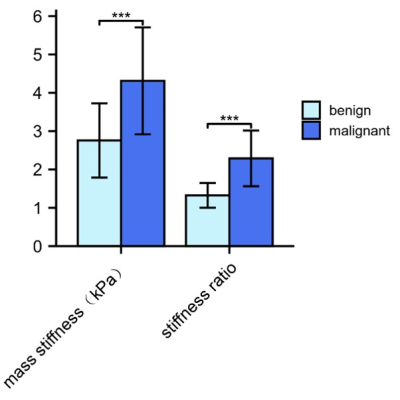
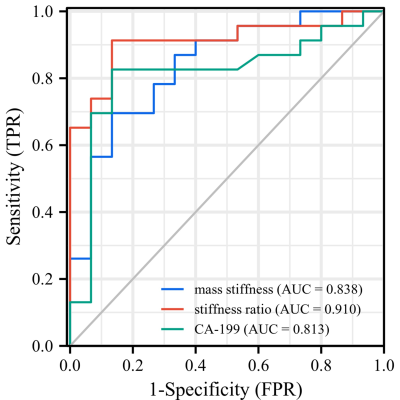
Figures 1 and 2 demonstrate two representative cases of benign pancreatic mass and PDAC in which solid tumors in similar subregions show apparently divergent stiffness values. Mean pancreatic masses stiffness of benign tumors and PDAC were 2.76±0.97kPa and 4.31±1.40kPa, showing significant difference (Student’s t test, P=0.001). There was no significant difference in parenchyma stiffness avoiding tumor and main pancreatic duct between benign masses (2.09±0.60) and PDAC (1.99±0.80kPa; Student’s t test, P=0.672). In terms of stiffness ratio, which was calculated by tumor stiffness/parenchyma stiffness, the value of PDAC (2.29±0.73) was significantly higher than that of benign masses (1.32±0.32; Student’s t test, P<0.001). Figure 3 demonstrates mass stiffness and stiffness ratio, which were significantly different between two groups. Figure 4 shows the AUC plots: stiffness ratio showed better diagnostic performance than that of mass stiffness and serum CA19-9 (AUC: 0.910 vs. 0.838 vs. 0.813).Discussion
Aforementioned results indicate that MRE-derived parameters are relatively superior than DWI-derived ADC value and serum CA19-9 level for differentiation between malignant and benign pancreatic solid tumors, with outstanding sensitivity, specificity, positive predictive value, negative predictive value, and accuracy. While further detailed clinical validation is needed, our study was able to provide encouraging evidence that the application of MRE will yield fruitful new achievements in comparison of conventional MRI. Further, the improvement of image quality, particularly the resolution can be expected in the near future.Conclusion
MR elastography-derived stiffness and stiffness ratio could serve as cogent imaging markers for non-invasive differentiation between PDAC and other benign pancreatic masses. With high predictive ability, these MRE-derived parameters have potential value in future clinical practice and assist in clinical decisions making.Acknowledgements
NoneReferences
1. Mizrahi, J. D.; Surana, R.; Valle, J. W.; Shroff, R. T. Pancreatic Cancer. The Lancet 2020, 395 (10242), 2008–2020. https://doi.org/10.1016/S0140-6736(20)30974-0.
2. Loomba, R.; Wolfson, T.; Ang, B.; Hooker, J.; Behling, C.; Peterson, M.; Valasek, M.; Lin, G.; Brenner, D.; Gamst, A.; Ehman, R.; Sirlin, C. Magnetic Resonance Elastography Predicts Advanced Fibrosis in Patients with Nonalcoholic Fatty Liver Disease: A Prospective Study. Hepatology 2014, 60 (6), 1920–1928. https://doi.org/10.1002/hep.27362.
3. Bohte, A. E.; de Niet, A.; Jansen, L.; Bipat, S.; Nederveen, A. J.; Verheij, J.; Terpstra, V.; Sinkus, R.; van Nieuwkerk, K. M. J.; de Knegt, R. J.; Baak, B. C.; Jansen, P. L. M.; Reesink, H. W.; Stoker, J. Non-Invasive Evaluation of Liver Fibrosis: A Comparison of Ultrasound-Based Transient Elastography and MR Elastography in Patients with Viral Hepatitis B and C. Eur Radiol 2014, 24.
4. 638–648. https://doi.org/10.1007/s00330-013-3046-0.(4) Tapper, E. B.; Loomba, R. Noninvasive Imaging Biomarker Assessment of Liver Fibrosis by Elastography in NAFLD. Nat Rev Gastroenterol Hepatol 2018, 15 (5), 274–282. https://doi.org/10.1038/nrgastro.2018.10.
Figures