0508
Ultra-High b-Value DWI in Predicting Progression risk of Locally Advanced Rectal Cancer: A Comparative Study with Routine DWI1Department of Radiology, Xijing Hospital, Xi’an, China, 2Department of MR Research, GE Healthcare China,, Beijing, China, 3Department of Pharmaceuticals Diagnostics, GE Healthcare China, Beijing, China
Synopsis
Keywords: Cancer, Diffusion/other diffusion imaging techniques
The prognosis prediction of locally advanced rectal cancer (LARC) was important to individualized treatment, we investigated the performance of ultra-high b-value DWI (UHBV-DWI) in progression risk prediction of LARC and compare with routine DWI. It was found that ADCuh derived from UHBV-DWI performed better than ADC based on routine DWI in predicting prognosis of LARC. The model based on combination of ADCuh, TNM-stage and extramural venous invasion (EMVI) could help to indicate progression risk before treatment.Introduction
It is crucial to make a precise prediction about the progression risk with the aim of individualized treatment. Analysis of routine DWI involving prognosis prediction have been investigated recently in rectal cancer [1-3] and colorectal cancer [4, 5]. However, routine DWI has not performed satisfactorily and has exhibited controversial results in prognosis prediction of rectal cancer [6]. Recently, ultra-high b-value DWI (UHBV-DWI) is increasingly explored in relation to the cerebral system [7-9] and prostate cancer [10, 11] and has showed considerable potential in tumor grading and detection. Thus, in this study, UHBV-DWI was introduced to evaluate the progression risk of locally advanced rectal cancer (LARC) and compare with routine DWI.Methods
PatientsThe institutional review board of Xijing hospital approved this retrospective study. Patients (n = 230) were consecutively recruited from November 2016 to May 2019 according to the inclusive criteria. The outcomes in this study were 3-year progression free survival (PFS). The date of last follow-up was June 30, 2021.
Multi-b value DWI acquisition
All patients underwent traditional DWI (b = 0, 1000 s/mm2) and multi-b-value DWI (b = 0~3500 s/mm2) on a 3.0 T MR scanner (Discovery MR750, GE Medical Systems) with a single-shot SE-EPI diffusion-weighted sequence. Other parameters of multi-b-value DWI were as follows: TR/TE = 4607/78.8 ms, FOV = 430×320 mm2, Matrix = 128×128, Slice thickness = 5 mm, Intersection gap = 0.5 mm and NEX = 4 to 8 (increasing with b-values).
ADC, ADCuh calculation and survival assessment
Routine DWI (b = 0, 1000s/mm2) was used to calculate ADC with VOI drawn at b1000 DWI and UHBV-DWI (b = 0, 1700~3500 s/mm2) was used to generate ADCuh with VOI drawn at b1700 DWI with mono-exponential model. Mean value of ADC and ADCuh were record for further analysis.
The performance of ADC and ADCuh in predicting prognosis was explored with time-dependent receiver operator characteristic curve (ROC) and Kaplan-Meier curves. Univariate and multivariate COX proportional hazard regression model was used to perform survival analysis and construct prognostic models for 3-year PFS prediction with clinicopathologic factor and functional parameter of DWI. The time-dependent ROC, decision curve analysis (DCA) and calibration curve was used to evaluate the discrimination, net benefit and agreement of prognostic models respectively.
Statistics
Statistical analyses were carried out with R software (version 4.1.2), and two-side P < 0.05 was considered statistically significant for all tests.
Results
Patient characteristicsA total of 112 patients with TNM-stage Ⅱ-Ⅲ were finally involved for analysis according to exclusion criteria. The median follow-up time was 41 (range: 2-55) months. The 3-year PFS of the whole cohort was 76%.
Survival analysis with ADC and ADCuh
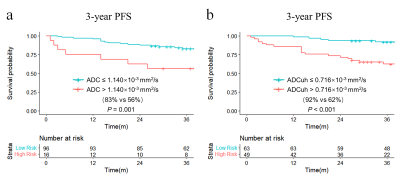
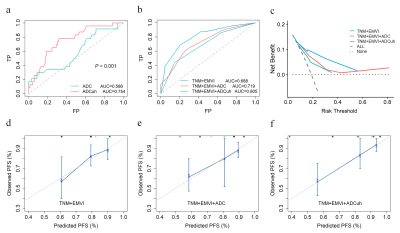
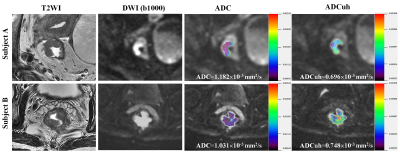
By using the time dependent ROC analysis, the optimal cutoff values of ADC and ADCuh were 1.140´10-3 mm2/s and 0.716´10-3 mm2/s according to patients 3-year PFS. The Kaplan-Meier curves exhibited significant difference between the low ADC group and high ADC group in 3-year PFS (83% vs 56%, P = 0.001, Figure 1a) as same as ADCuh (92% vs 62%, P < 0.001, Figure 1b). Time-dependent ROC showed that ADCuh was superior to ADC in 3-year PFS assessment (AUC = 0.754 vs 0.586, P < 0.001, Figure 2a). MR images of patients with proregression and without progression during follow-up was shown in Figure 3.
Prognostic model construction
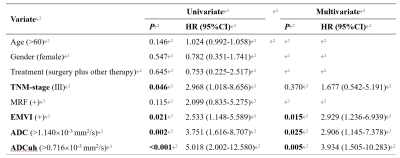
Univariate and multivariate COX analysis found that EMVI, ADC and ADCuh were the independent factors for 3-year PFS (Figure 4). We constructed three prognostic models for 3-year PFS assessment, model 1 (TNM +EMVI), model 2 (TNM+EMVI+ADC) and model 3 (TNM+EMVI+ADCuh). The model 3 has better performance than model 1 and model 2 (AUC = 0.805, 0.719, 0.688, respectively, Figure 2b). Decision curve (Figure 2c) exhibited that patients might have higher net benefit than model 1 and model 2. Meanwhile, calibration curves (Figure 2d-f) showed better agreement of model 3 between predicted PFS and observed PFS than other two models.
Discussion
This study found the ADCuh derived from UHBV-DWI performed better than ADC derived from routine DWI for 3-year PFS assessment in LARC (AUC = 0.754 vs 0.586) and combined model (TNM+EMVI+ADCuh) has good discrimination, net benefit and agreement for 3-year PFS prediction (AUC = 0.805).Though previous studies have demonstrated ADC was correlated with local recurrence or distance metastasis [1, 3] and disease-free survival [3], we found the performance of ADC was inferior to ADCuh in assessing PFS. Theoretically, when signal attenuation arrives at ultra-high b-value region, the sensitivity to smaller spatial scale enhances and enables DWI to explore tissue microstructure on complexity and heterogeneity more powerfully than routine DWI [12]. In fact, previous studies have showed that UHBV-DWI performed better than traditional DWI in tumor grading [8] and detection [10].
The combined model we constructed with ADCuh needs to be investigated and validated in additional datasets and future randomized controlled trials.
Conclusions
ADCuh based on UHBV-DWI is an independent prognosis factor for 3-year PFS of LARC and performed better than ADC from routine DWI. The combined model (ADCuh+TNM-stage+EMVI) could be a promising tool for progression risk prediction.Acknowledgements
We thank Prof. Lei Shang for suggestions about statistical analyses.References
1. Moon SJ, Cho SH, Kim GC, Kim WH, Kim HJ, Shin KM, Lee SM, Park JS, Choi GS, Kim SH: Complementary value of pre-treatment apparent diffusion coefficient in rectal cancer for predicting tumor recurrence. Abdominal radiology 2016, 41(7):1237-1244.
2. Bakke KM, Hole KH, Dueland S, Groholt KK, Flatmark K, Ree AH, Seierstad T, Redalen KR: Diffusion-weighted magnetic resonance imaging of rectal cancer: tumour volume and perfusion fraction predict chemoradiotherapy response and survival. Acta oncologica 2017, 56(6):813-818.
3. Noda Y, Goshima S, Kajita K, Kawada H, Kawai N, Koyasu H, Matsuo M, Bae KT: Prognostic Value of Diffusion MR Imaging and Clinical-Pathologic Factors in Patients with Rectal Cancer. Iran J Radiol 2018, 15(1):e57080.
4. Alis D, Durmaz ESM, Gulsen F, Bas A, Kabasakal L, Sager S, Numan F: Prognostic value of ADC measurements in predicting overall survival in patients undergoing (90)Y radioembolization for colorectal cancer liver metastases. Clinical imaging 2019, 57:124-130.
5. Takahashi Y, Hayano K, Ohira G, Imanishi S, Hanaoka T, Watanabe H, Hirata A, Kawasaki Y, Miyauchi H, Matsubara H: Histogram Analysis of Diffusion-Weighted MR Imaging as a Biomarker to Predict Survival of Surgically Treated Colorectal Cancer Patients. Digestive diseases and sciences 2021, 66(4):1227-1232.
6. Schurink NW, Lambregts DMJ, Beets-Tan RGH: Diffusion-weighted imaging in rectal cancer: current applications and future perspectives. The British journal of radiology 2019, 92(1096):20180655.
7. Xueying L, Zhongping Z, Zhoushe Z, Li G, Yongjin T, Changzheng S, Zhifeng Z, Peihao C, Hao X, Li H: Investigation of Apparent Diffusion Coefficient from Ultra-high b-Values in Parkinson's Disease. European radiology 2015, 25(9):2593-2600.
8. Tan Y, Zhang H, Wang XC, Qin JB, Wang L: The value of multi ultra high-b-value DWI in grading cerebral astrocytomas and its association with aquaporin-4. The British journal of radiology 2018, 91(1086):20170696.
9. Bai Y, Liu T, Chen L, Gao H, Wei W, Zhang G, Wang L, Kong L, Liu S, Liu H et al: Study of Diffusion Weighted Imaging Derived Diffusion Parameters as Biomarkers for the Microenvironment in Gliomas. Frontiers in oncology 2021, 11:672265.
10. Cha SY, Kim E, Park SY: Why Is a b-value Range of 1500-2000 s/mm(2) Optimal for Evaluating Prostatic Index Lesions on Synthetic Diffusion-Weighted Imaging? Korean journal of radiology 2021, 22(6):922-930.
11. Woo S, Suh CH, Kim SY, Cho JY, Kim SH: Head-To-Head Comparison Between High- and Standard-b-Value DWI for Detecting Prostate Cancer: A Systematic Review and Meta-Analysis. AJR American journal of roentgenology 2018, 210(1):91-100.
12. Tang L, Zhou XJ: Diffusion MRI of cancer: From low to high b-values. Journal of magnetic resonance imaging : JMRI 2019, 49(1):23-40.
Figures