0507
Slice Level 3D Motion Tracking For Motion Corrected IVIM DW-MRI In Crohn’s Disease
SERGE DIDENKO VASYLECHKO1, LINA LU1, CEMRE ARIYUREK1, JEANNETTE PEREZ-ROSSELLO1, MICHAEL CALLAHAN1, ONUR AFACAN1, and SILA KURUGOL1
1RADIOLOGY, BOSTON CHILDREN'S HOSPITAL, HARVARD MEDICAL SCHOOL, BOSTON, MA, United States
1RADIOLOGY, BOSTON CHILDREN'S HOSPITAL, HARVARD MEDICAL SCHOOL, BOSTON, MA, United States
Synopsis
Keywords: Digestive, Motion Correction
Diffusion-weighted MRI is increasingly used for detection and characterization of Crohn’s disease. However, unavoidable respiratory motion and bowel motility reduces accuracy and precision of quantitative parameter fitting, which hinders clinical applicability DW-MRI. We use a 3D slice-to-volume registration approach that sequentially tracks rigid motion parameters for each slice and regularises the parameters with a Kalman filter in the order of acquisition of each slice. We assess the quality of images and estimated parameter maps and the precision of IVIM parameters in the areas of disease using the proposed motion correction technique, and compare them with results from the uncorrected data.Introduction
Diffusion-weighted MRI signal is increasingly used in assessment of Crohn’s disease (CD) owing to improvements in MRI hardware1. In comparison to the single exponential signal decay model, bi-exponential intravoxel incoherent motion model (IVIM) can be used from multi-b-value DW-MRI, which provides a more accurate slow diffusion decay parameter (D) and additional fast diffusion coefficient fraction (f) parameter2.The model is given by:
$$S(b)=S_{0}\left(f e^{-b D^{*}}+(1-f) e^{-b D}\right)$$
where D* is fast diffusion parameter, S0 is non-diffusion dependent signal, and b is b-values vector.
IVIM parameters can be used for detection of lesions and characterization of inflammation and fibrosis3. Nevertheless, due to unavoidable respiratory motion and motility of bowel, the accuracy and precision of the quantitative parameter fitting is reduced, which hinders clinical applicability. Respiratory motion can be compensated using respiratory triggering, however this method does not correct for bowel motility. Previous methods used non-rigid registration and model based registration for motion correction in abdominal DW-MRI4-6. However limitations persist due to the ill-posedness of the non-rigid registration. Here we use a 3D slice-to-volume registration (SVR) approach that sequentially tracks rigid motion parameters for each slice and regularises the parameters with a Kalman filter along the order of slice acquisition7-8. We assess the quality of images and parameter maps and the precision of IVIM parameters in the areas of CD using the proposed motion correction technique, and compare them with results from the uncorrected data.
Methods
DW-MR images from 11 pediatric Crohn's disease patients were acquired clinically at 3T (MAGNETOM Prisma, Siemens) using a free-breathing single-shot EPI: TR/TE=8100/67;matrix=156x120;FOV=340x260mm;slice-thickness=5mm;b-values=0,20,50,100,200,400,600,800s/mm2;6 gradient directions;acquisition-time= 6.5min. Images were retrospectively reviewed according to the IRB protocol. Post-contrast T1 and DW-MRI were assessed by a trained radiologist and CD regions were segmented.Six parameters of 3D rigid motion for each slice were first estimated using 3D SVR. The slice level motion tracking based on Kalman filtering was then used to regularise motion parameters for each slice in the order of acquisition.
First, b=0s/mm2 image with least amount of motion was used as the initial reference volume. To prevent motion estimates to skew towards non specific regions of interest, the volume was cropped around the Crohn’s disease area. Next, motion parameters for each slice in the b=0 volumes were sequentially estimated by registering the reference volume to each slice.Then, updated average B0 image was reconstructed. This averaged B0 image is then used as reference to register the next set of b values, and the process is repeated for all b-values.
Estimated motion parameters were applied to each slice for motion correction using a scattered point cloud interpolation scheme, which uses a regular reconstruction grid to map a collection of Gaussian kernel weighted voxels from motion space. The entire motion estimation and reconstruction algorithm was written in C++ with ITK and ANIMA9,10, and is available on github.com/quin-med-harvard-edu/dSVRK, and as a docker image hub.docker.com/r/quinlab/dSVRK. Next, IVIM model was fitted to uncorrected and motion corrected data for estimation of D and f parameters using DIPY library11.
To assess improvement in precision of estimated parameters after motion correction, bootstrap analysis was used. Random removal of gradient directions in the original data was performed to generate subsampled data points at each bootstrap iteration. In total 61 iterations were performed. IVIM model was repeatedly fit to each bootstrap subsampled signal. Evaluation consisted of the coefficient of variation estimation of the D and f parameters over multiple bootstrap iterations.
Results
Figure 1 shows the quality of IVIM model parameter maps before and after motion correction. The image quality of the parameter maps improved significantly after motion correction in all subjects.Figure 2 shows the quality of b=50,400,800s/mm2 images before and after motion correction in 3 orthogonal planes. The areas with motion, the structural details in the images and the consistency of the anatomy were all improved after motion correction.
Figure 3 shows a line of voxels plotted over time in the order of slice acquisition for all volumes of an example subject. If there was no motion, straight lines would appear across time. Motion corrected data showed more consistent lines over time compared to uncorrected data.
Figure 4 shows the results from the assessment of precision of IVIM parameter estimates via bootstrap analysis on the entire dataset. Motion corrected data exhibits significant reduction in CoV of parameters, which is driven by significant reduction in standard deviation of parameter values after alignment of multiple b-value images.
Figure 5 shows improved anatomical consistency between T1 images and DW-MRI after motion correction.
Conclusions and Discussion
The proposed 3D slice level motion tracking method could correct for the motility and respiratory related motion of Crohn’s disease regions in DW-MRI. Motion correction significantly improved precision of IVIM parameters by reducing the CoV% of the estimated IVIM parameters. The motion corrected images and the resultant parameter maps all showed improved image quality, improving the consistency of structures and reducing the misalignments in both spatial and temporal dimensions. Despite the nonrigid nature of motion, a local 3D rigid SVR method that tracked the 3D rigid motion of each slice was effective in correcting motion in DW-MRI of Crohn’s disease.Acknowledgements
This work was supported in part by NIH grants R01 EB019483, R01 NS121657, R01 DK125561, R21 DK123569, R21 EB02962, and a pilot grant (PP-1905-34002) from the National Multiple Sclerosis Society.References
- Dohan, Anthony, et al. "Diffusion‐weighted MRI in Crohn's disease: current status and recommendations." Journal of Magnetic Resonance Imaging 44.6 (2016): 1381-1396.
- M. Freiman, et al. Characterization of fast and slow diffusion from diffusion‐weighted MRI of pediatric Crohn's disease. Journal of Magnetic Resonance Imaging 37.1 (2013): 156-163.
- Kurugol, Sila, et al. Evaluation of Motion-Compensated Spatially-Constrained IVIM (MC-SCIM) Model of Diffusion-weighted MRI for Assessment of Fibrosis in Crohn’s Disease using Surgical Histopathology Scores. ISMRM 2017.
- J.M. Guyader, et al. "Influence of image registration on apparent diffusion coefficient images computed from free‐breathing diffusion MR images of the abdomen." Journal of Magnetic Resonance Imaging 42.2 (2015): 315-330.
- S. Kurugol, et. al. Motion-robust parameter estimation in abdominal diffusion-weighted MRI by simultaneous image registration and model estimation, Medical Image Analysis, 39, 124-132, 2017.
- J. Zaffrani-Reznikov, et al. qDWI-Morph: Motion-compensated quantitative Diffusion-Weighted MRI analysis for fetal lung maturity assessment. arXiv preprint arXiv:2208.09836, 2022.
- J. Coll‐Font, et al. Retrospective Distortion and Motion Correction for Free‐Breathing DW‐MRI of the Kidneys Using Dual‐Echo EPI and Slice‐to‐Volume Registration. Journal of Magnetic Resonance Imaging, 2020.
- S. Kurugol, et al. Motion-Robust Spatially Constrained Parameter Estimation in Renal Diffusion-Weighted MRI by 3D Motion Tracking and Correction of Sequential Slices, MICCAI RAMBO workshop, Lecture Notes in Computer Science, vol 10555. Springer, 2017.
- M. McCormick, et al. ITK: enabling reproducible research and open science. Front Neuroinform. 8:13, 2014.
- O. Commowick, et al. Automated diffeomorphic registration of anatomical structures with rigid parts: application to dynamic cervical MRI. MICCAI, pp.163-70, 2012.
- E. Garyfallidis, et al. DIPY, a library for the analysis of diffusion MRI data. Frontiers in Neuroinformatics, 8.8, 2014.
Figures
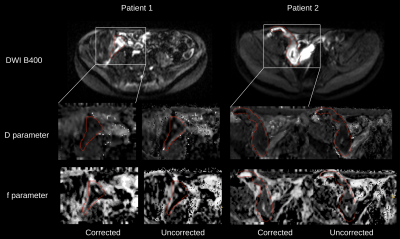
Visual evaluation of Crohn's disease in the bowel on the estimated IVIM D and f parameter maps, and the corresponding free-breathing (uncorrected) b=400s/mm2 image. A white bounding box indicates the region from which the subimages were derived. A contour of the Crohn’s disease was segmented by a trained radiologist as shown in red. Both D and f parameters of the motion corrected data (i.e. corrected) contain a more distinct structure and homogeneous appearance around the disease region compared to parameters from the original data (i.e. uncorrected).
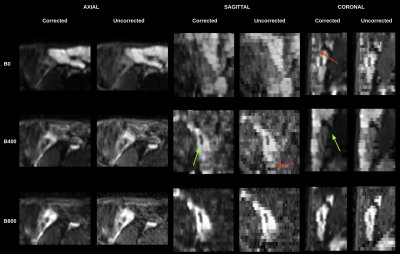
An example of motion corrected and original (uncorrected) data for b=50,400,800s/mm2 images, shown in three orthogonal planes for the Crohn’s disease region labelled by the radiologist for Patient 1 (from Fig 1). Red arrows indicate the areas of motion that had been improved with the proposed 3D slice level motion correction technique resulting in a more consistent 3D anatomy in all planes. The reconstruction yields an increase in the resolution in the through-slice direction, from 5mm to 1.3mm, which improves the structural details, as shown by green arrows.
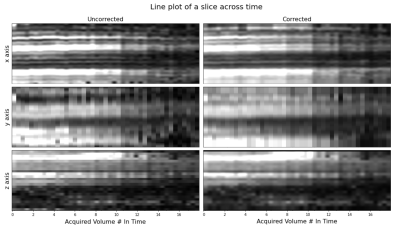
A line of voxels taken from a middle slice in each of the three respective planes and plotted over time in the order of slice acquisition for all volumes of an example subject. In case of no motion, straight lines would appear across time. Motion corrected data exhibits reduced variation in motion as seen shown in this line plot.
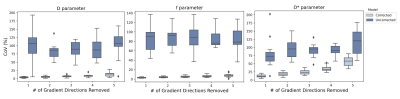
Assessment of precision of IVIM parameters via bootstrap analysis on all subjects. Randomly chosen gradient directions were removed from signal multiple times, followed by IVIM parameter estimation. CoV of estimated parameters were calculated from mean estimates of each parameter in the diseased region of each subject. Boxplots show mean and standard deviation of CoV over 10 subjects. Motion corrected data exhibits significant reduction in CoV. This result is consistent with the more consistent appearance of parameter maps in Crohn’s disease regions that were shown in Figure 1.
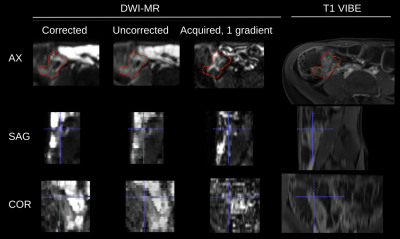
Anatomically correspondent T1 Radial VIBE and DW-MR images, with indications of a lesion (red segmentation) and a spatially matching marker (blue cross hair). DW-MR images include: motion corrected geometrically averaged B400 image, free-breathing (uncorrected) geometrically average B400, a single gradient acquisition of the B800 image (before geometric averaging). Motion corrected images exhibit sharper structural details, such as the region under cross hair in the sagittal and coronal views of DW-MR, that correspond better to the anatomy shown on T1 data.
DOI: https://doi.org/10.58530/2023/0507