0500
Toward the Use of MRS Methodological Consensus by the Clinical Research Community - An Early Assessment of Dissemination Results1Department of Psychiatry and Behavioral Health, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, United States, 2New York State Psychiatric Institute and Department of Psychiatry, Columbia University Irving Medical Center, New York City, NY, United States, 3Department of Neurology, University of Kansas Medical Center, Kansas City, KS, United States, 4Hoglund Biomedical Imaging Center, University of Kansas Medical Center, Kansas City, KS, United States, 5Magnetic Resonance Methodology, Institute of Diagnostic and Interventional Neuroradiology, University of Bern, Bern, Switzerland, 6Translational Imaging Center, sitem-insel, Bern, Switzerland, 7Department of Biomedical Engineering, Columbia University School of Engineering and Applied Science, New York, NY, United States, 8Department of Radiology, Columbia University Irving Medical Center, New York, NY, United States
Synopsis
Keywords: Spectroscopy, Translational Studies
The MRS community recently established a set of expert Consensus Recommendations. To date, however, knowledge on their implementation beyond anecdotal reports is largely lacking. Here we report a preliminary assessment of the achieved dissemination and, more importantly, the level of acceptance of the established guidelines and standards by the clinical MRS research community. We compiled feedback from 22 editors-in-chief of major clinical journals, together accounting for more than 100 (>12%) of the in vivo clinical MRS publications from 2021.Introduction
In vivo magnetic resonance spectroscopy (MRS) enables localized measurement of diverse biochemicals in the living human body. However, the potential of MRS in research and clinical diagnostics has not been fully realized, in part due to a lack of consensus on methodology and reporting standards, which has rendered the objective assessment, comparison, and reproduction of results difficult.The international MRS community recently joined forces, represented by the expert MRS Consensus Group, to create a comprehensive overview of in vivo MRS methodology and specific recommendations for its use in biomedical research, in line with similar efforts in other clinical fields (1-3). This effort produced 13 recommendation papers in a special issue of NMR in Biomedicine edited by Drs. Choi and Kreis (4), including a summary of minimum reporting standards (MRSinMRS, Figure 1).
Aside from anecdotal reports, however, the level of awareness and degree of practical implementation across the scientific community - in particular, the clinical research community - remains unknown. Here, we assess the impact of the MRS consensus effort in the clinical research community.
Methods
We set out to characterize acceptance of the MRS Consensus Guidelines by clinical journal editors - the gatekeepers of publication - through an e-letter introducing the consensus effort and inquiring about an editor’s awareness of these recommendations, the journal’s current method for standardizing MRS reporting, and willingness to support implementation (Figure 2).We conducted a systematic literature search to identify the clinical journals with significant impact publishing in vivo MRS (Figure 3). Using publisher-neutral electronic databases, Journal Citation Reports (JCR) and Web of Science (WOS), we extracted clinical journals with impact factors (JIF) ≥2 in 2021, and then extracted the MRS papers in English published in these journals in 2021. Output articles were sorted by journal and by category to confirm broad representation of clinical specialties (Figure 4). Target journals included those publishing ≥2 MRS studies, supplemented by journals with ≥1 MRS reports for underrepresented clinical specialties and additional journals of known relevance.
For each of the target journals, we identified the email address(es) of the editor(s)-in-chief, sent the e-letter, and catalogued responses with respect to each editor’s reception to the MRS consensus guidelines.
Results
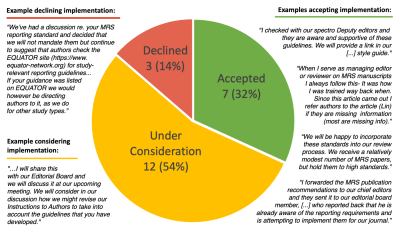
The JCR search yielded 2362 clinical journals and the WOS keyword search within these journals identified 838 MRS papers in 368 journals. After filtering and supplementing (8 journals with only 1 published paper and 10 additional suggestions), emails were sent to editors-in-chief of 123 journals, which together accounted for 537 (64%) of the clinical MRS papers from 2021 and a broad spectrum of clinical specialties (Figures 3/4).We received feedback from 22 (18%) of the editors solicited, accounting for 109/838 (13%) of the clinical MRS papers (Figure 5). Of the 22 responses, 19 editors (101/838=12% of papers) were supportive, suggesting implementation was widely acceptable. A subset of 6 editors (30/838=4%) reported intention to implement the MRSinMRS guidelines, while 13 editors (71/838=8%) reported implementation would require additional consideration, six of whom had already started discussion with their editorial boards.
Of the 3 editors who declined implementation, two (5/838=1%) stated that authors are referred to the EQUATOR website (5) for reporting guidelines for health science research. The third editor explained that her journal (3/838<1%) does not seek to publish in vivo MRS.
Preliminary results of this work were presented at the MRS Workshop in Lausanne, Switzerland, in August 2022. Notably, there had been limited implementation of the agreed-upon standardized tabular format proposed in (6), even among MRS experts who contributed to the standards. Additional remarks addressed the burden of creating the table, and the benefits of top-down vs. bottom-up approaches to dissemination, with trainees advocating for the latter.
Discussion
We assessed the acceptance level of the recently established MRS Consensus Recommendations by the clinical MRS community. We chose to approach the editors-in-chief due to their central role in the publishing process. We received feedback from editors of 22 (18%) of journals contacted and 19 (86%) of these responses were supportive, representing 101 (>12%) of the total MRS papers published in clinical journals in 2021.Our response rate of 18% is respectable as ~10% is typical from email solicitation campaigns (7,8). Notably, this promotion effort is expected to increase awareness even if journal editors did not respond.
For those editors who responded ‘under consideration’, many mentioned discussion with the journal’s editorial board, or even an overarching management board, as a requisite step. The importance of the editor’s advocacy appears key for broad acceptance and implementation of the guidelines. Moreover, personal contacts might be crucial, based on our subjective impression that many of our responses came from such journal editors.
The EQUATOR website could enhance the further situation of our standards within the larger health sciences community. Like every literature search, some level of type I error (leaving out relevant journals) and type II error (inclusion of irrelevant journals, e.g. ex vivo) is expected.
This study provides a snapshot of the acceptance level of the MRS Consensus Recommendations. It has yet to be seen whether these standards will be further implemented by publishers and authors. The presented methods set the stage for ongoing monitoring, targeted promotion, and improved dissemination.
Acknowledgements
We thank the editors who provided feedback, as well as the MRS Consensus Working Group and the 2022 MRS Workshop in Lausanne for their initiative and support.
This work was supported by grants K23 MH115291 and R01 MH126133 from the National Institutes of Health.
References
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. J Pharmacol Pharmacother. 2010;1(2):100-7. Epub 2011/02/26. doi: 10.4103/0976-500X.72352. PMID: 21350618; PMC3043330.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;372:n71. Epub 2021/03/31. doi: 10.1136/bmj.n71. PMID: 33782057; PMC8005924
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus Nomenclature for in Vivo Imaging of Reversibly Binding Radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533-9. PMID: 17519979.
- Choi IY, Kreis R (Editors). Special Issue: Advanced Methodology for in Vivo Magnetic Resonance Spectroscopy. NMR Biomed. 2021;34(5). doi: 10.1002/nbm.4334.
- EQUATOR Network, https://www.equator-network.org.
- Lin A, Andronesi O, Bogner W, Choi IY, Coello E, Cudalbu C, Juchem C, Kemp GJ, Kreis R, Krššák M, Lee P, Maudsley AA, Meyerspeer M, Mlynarik V, Near J, Öz G, Peek AL, Puts NA, Ratai EM, Tkáč I, Mullins PG, Experts' Working Group on Reporting Standards for MRS. Minimum Reporting Standards for in Vivo Magnetic Resonance Spectroscopy (MRSinMRS): Experts' Consensus Recommendations. NMR Biomed. 2021;34(5):e4484. Epub 2021/02/10. doi: 10.1002/nbm.4484. PMID: 33559967; PMC8647919.
- Martin S. How We Increased Email Reply Rate: MOZ.com. 2017. [Internet: cited 10/28/2022]. Available from: https://moz.com/blog/how-we-increased-email-reply-rate.
- Hutchings R. When Doing Email Blogger Outreach What Response Rate Do You Expect? MOZ.com. 2011. [Internet: cited 10/28/2022]. Available from: https://moz.com/community/q/topic/9104/when-doing-email-blogger-outreach-what-response-rate-do-you-expect.
Figures
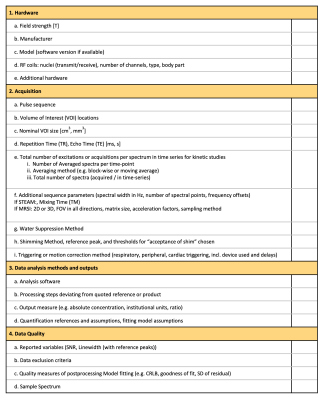
FIGURE 1. MRSinMRS Checklist. List of minimum reporting standards for the reporting of MRS methods and results, including the standardized description of MRS hardware, data acquisition, analysis, and quality assessment adapted from Table 1 in Lin et al. (2021) (6).
T=Tesla, RF=RadioFrequency, STEAM=STimulated Echo Acquisition Mode, MRSI=Magnetic Resonance Spectroscopic Imaging, FOV=Field of View, SNR=Signal-to-Noise Ratio, CRLB=Cramer-Rao Lower Bound

Figure 2. E-letter to Editors. Replication of e-letter sent to editors of 123 journals in 2022. The text included live links to papers (on the journal publisher’s site) and the MRSinMRS checklist [Fig. 1 herein].
CONSORT=CONsolidated Standards Of Reporting Trials, PRISMA=Preferred Reporting Items for Systematic Reviews and Meta-Analyses
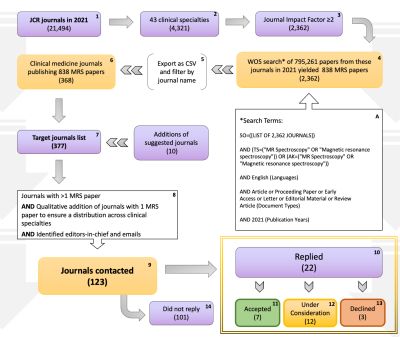
Figure 3. Flow Chart. Depicts the 10-step process used to identify journal editors to whom e-letter was sent. It details the search for journals (# of journals after each step) across clinical specialties [2], filtered by impact factor [3], and publishing MRS data reports in English in 2021 [4]. After sorting, filtering & supplementing [7,8], the editors-in-chief of 123 journals were identified & contacted [9], and editors’ replies were grouped qualitatively [10-13].
JCR=Journal Citation Reports, WOS=Web of Science, MRS=Magnetic Resonance Spectroscopy
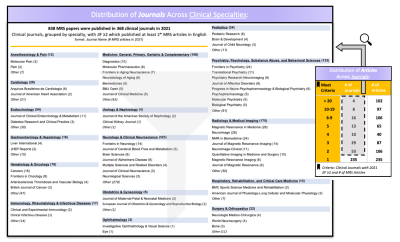
Figure 4. Journal Search Results. Numbers of MRS articles published in 2021 for each specialty are shown in bold, along with a sampling of journals in each. Journals were generally included in the target list if they published ≥2 MRS papers in 2021. *For some specialties, journals publishing only 1 MRS article were included to ensure representation of specialties. In this figure, some journals and therefore their articles are counted in multiple clinical categories.
JIF=Journal Impact Factor