0493
Use of fast B1 shimming to enable localized MR spectroscopy in different regions of the human brain on a clinical platform at 7 Tesla1Biomedical Engineering, King's College London, London, United Kingdom, 2London Collaborative Ultra high field System (LoCUS), King's College London, London, United Kingdom, 3System Technologies, Siemens Healthcare GmbH, Erlangen, Germany, 4Lysholm Department of Neuroradilogy, National Hospital for Neuroradiology and Neurosurgery, London, United Kingdom, 5MR Research Collaborations, Siemens Healthiness, Frimley, United Kingdom, 6Radiology, Great Ormond Street Hospital, London, United Kingdom, 7Center for Stroke Research Berlin, Charité Universitätsmedizin Berlin, Berlin, Germany
Synopsis
Keywords: Spectroscopy, High-Field MRI, Paralel Transmit and B1 shimming
We demonstrated that applying a fast implementation of an RF phase shimming method enabled the acquisition of localized MR spectra in different regions of the human brain including B1 challenging areas, such as the hippocampus and prefrontal cortex, using a commercially available pTx coil on a clinical platform at 7T. RF efficiency was increased by ~100% cerebellum and PFC while ~50-75% for pons and hippocampus. The most favorable flip angle distributions were obtained for the cerebellum and the prefrontal cortex. Fast B1 shimming with advanced MRS methodology enables high-quality single voxel spectra at 7T using a commercially available setup.Introduction
Magnetic Resonance Spectroscopy (MRS) is a non-invasive technique to quantitatively investigate the underlying molecular mechanisms of pathological changes in various organs, in particular in the human brain[1]. Reaping the twofold benefits of its application on 7T MRI scanners, enhanced sensitivity and increased spectral resolution, is often limited by increased radiofrequency (RF) inhomogeneity and higher SAR values that indicate tissue heating. However, modern 7T scanners are equipped with parallel-transmit (pTx) systems that enable dynamic control of RF signal amplitude and phase to mitigate any such limitations. So far, localized MRS facilitating B1-shimming across different regions of the human brain at 7T has only been demonstrated using a customized setup including home-built RF coils[2,3]. Alternatively, dielectric pads, where placement has to be adjusted for each individual subject, have been used to improve B1 transmission at this field strength[4]. In this work, it was demonstrated that applying a fast implementation of an RF phase shimming method enabled the acquisition of localized MR spectra in different regions of the human brain including B1 challenging areas, such as the hippocampus and prefrontal cortex, using a commercially available pTx coil on a clinical platform at 7T.Methods
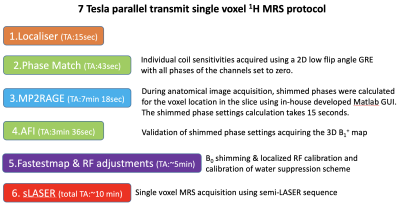
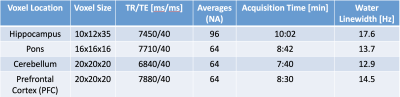
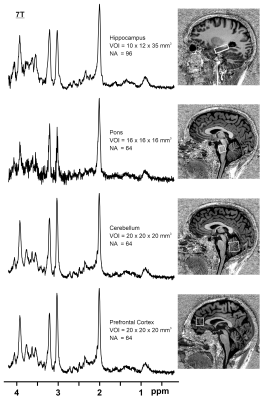
Data collection:MRI/MRS data were collected in 6 healthy volunteers (5M/1F, mean-age 39-years) on a 7T Terra parallel-transmit (pTx) scanner (Siemens Heathineers, Erlangen, Germany) using the standard 32-channel receive/8-channel transmit pTx head coil (Siemens Healthineers/Nova Medical Inc.,MA). All components of the MRI/MRS protocol are summarized in a flowchart (Fig.1). A fast B1 shimming acquisition(~40seconds) with 2D low-flip angle GRE image for the slice containing the MRS voxel. Its raw data were exported and RF phase shim settings to increase the RF efficiency in the voxel localization were calculated for 15 seconds using an in-house developed optimisation MATLAB GUI (The MathWorks Inc.,MA) to find phase shim settings applied on the system prior to all subsequent MRS acquisitions [5]. Shimmed RF settings in different brain regions were validated with 3D-AFI flip angle maps[6]. B0 shimming was performed using FAST(EST)MAP[7] followed by localized RF adjustments. Single voxel MRS data were then acquired in the hippocampus (N=2), pons (N=2), cerebellum (N=1); and prefrontal cortex (PFC, N=2) using a semi-LASER [3,8] (TE=40 ms, spectral width = 6000 Hz, vector size = 4096, frequency offset =-2.0 ppm) with VAPOR water suppression[9] and outer volume saturation. Region-specific scan parameters are shown in Table1, note that the minimum TR that passed the SAR limits was used for each voxel, which was always larger than 6000 ms.Data analysis: MR spectra were pre-processed including frequency drift using the FID-A toolkit[10] and eddy current correction. Metabolite quantification of the resulting spectra was performed using LCModel[11] with a simulated basis set. T1-weighted MP2RAGE images were segmented using SPM12, and voxel tissue fractions were calculated with FSL to correct all metabolite concentrations for the CSF content of each voxel.
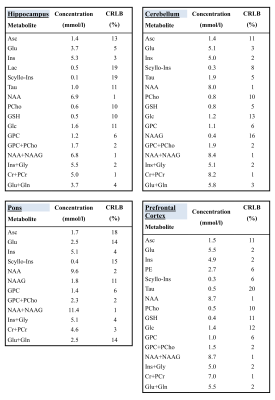
Results
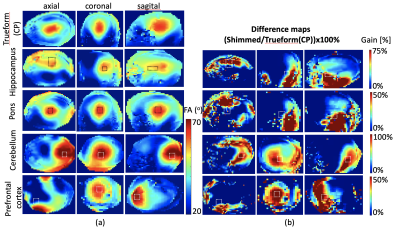
Results from B0 shimming are included in Table1. Fig.2a shows resulting flip angle maps of the standard single-transmit system and Trueform mode of the pTx system and the B1-shimmed flip angle maps for hippocampus, pons, cerebellum and prefrontal cortex. The difference maps show the percentage difference of the shimmed and trueform cases for the slices of the voxel location (Fig.2b). For all pTX cases, a substantial gain in RF efficiency is visible compared to a single-transmit setup (trueform). RF efficiency was increased by 50-100%. This enabled acquisitions of single voxel sLASER data from brain regions within SAR limits and a clinically acceptable/feasible acquisition time within 10 minutes. Note the overall high spectral quality for all regions, where differences in SNR can be largely attributed to different voxel sizes (Fig.3). A neurochemical profile of, at least 7 individual and 5 combined metabolites was reliably determined in each region (Table1) (Cramer-Rao Lower Bounds(CRLBs) below 25% were retained). Metabolite levels were within the range of literature values (Table2) [2,3,12].Discussion
Use of a standard parallel-transmit system equipped with a commercially available RF-coil array allowed single voxel MRS acquisitions at 7T after application of a fast in-house developed RF phase shimming algorithm. Due to the resulting increased RF efficiency/B1 transmit fields, high quality localized MR spectra were obtained from different brain regions. Without the applied B1-shimming method, MRS data acquisitions would not have been feasible in the selected brain regions. While RF phase shimming in the cerebellum and PFC were largely optimal with the available pTx coil, the pons and hippocampus RF phase shimming was suboptimal as can be seen from the corresponding FA maps(Fig.2). This is due to the fact that the capability to locally increase B1 transmit fields strongly depends on the geometry of the given pTx channels. For proof of feasibility the minimum TR that was in-line with SAR restrictions was chosen for each MRS scan. Further improvements in RF efficiency would lower this minimum required TR. In larger cohorts, the same TR should be selected for all subjects.Conclusion
Fast B1-shimming combined with advanced MRS methodology yielded the acquisition of high-quality single voxel spectra in the human brain at 7T using a commercially available setup. Further work will include application to additional brain regions and automisation of the entire setup towards usage as a clinical tool.Acknowledgements
This work was supported by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. The MRS package was developed at the University of Minnesota (by Gulin Oz, Ivan Tkac, Dinesh Deelchand, Edward J. Auerbach and Malgorzata Marjanska) and was provided within C2P agreement. We are also grateful to Dinesh Deelchand and Malgorzata Marjańska a for sharing metabolites basis-sets for LCM analysis.References
[1] Öz G, Deelchand DK, Wijnen JP, et al. Advanced single voxel 1H magnetic resonance spectroscopy techniques in humans: Experts’ consensus recommendations. NMR Biomed. 2021;34(5):1-18. doi:10.1002/nbm.4236
[2] Emir UE, Auerbach EJ, Van De Moortele PF, Marjańska M, Uğurbil K, Terpstra M, Tkáč I, Oz G. Regional neurochemical profiles in the human brain measured by ¹H MRS at 7 T using local B₁ shimming. NMR Biomed. 2012 Jan;25(1):152-60. doi: 10.1002/nbm.1727. Epub 2011 Jul 15. PMID: 21766380; PMCID: PMC3197892.
[3] Marjańska M, Auerbach EJ, Valabrègue R, Van de Moortele PF, Adriany G, Garwood M. Localized 1H NMR spectroscopy in different regions of human brain in vivo at 7 T: T2 relaxation times and concentrations of cerebral metabolites. NMR Biomed. 2012 Feb;25(2):332-9. doi: 10.1002/nbm.1754. Epub 2011 Jul 27. PMID: 21796710; PMCID: PMC3357544.
[4] Snaar JE, Teeuwisse WM, Versluis MJ, van Buchem MA, Kan HE, Smith NB, Webb AG. Improvements in high-field localized MRS of the medial temporal lobe in humans using new deformable high-dielectric materials. NMR Biomed. 2011 Aug;24(7):873-9. doi: 10.1002/nbm.1638. Epub 2010 Dec 28. PMID: 21834010.
[5] Clément J, Tomi-Tricot R, Malik SJ, Webb A, Hajnal JV, Ipek Ö. Towards an integrated neonatal brain and cardiac examination capability at 7 T: electromagnetic field simulations and early phantom experiments using an 8-channel dipole array. Magn Reson Mater Phys Biol Med. 2022 Oct 1;35(5):765–78.
[6] Yarnykh VL. Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn Reson Med. 2007 Jan;57(1):192–200.
[7] Gruetter R, Tkác I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000 Feb;43(2):319-23. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. PMID: 10680699.
[8] Scheenen TW, Klomp DW, Wijnen JP, Heerschap A. Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magn Reson Med. 2008 Jan;59(1):1-6. doi: 10.1002/mrm.21302. PMID: 17969076.
[9] Tkác I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999 Apr;41(4):649-56. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. PMID: 10332839.
[10] Simpson R, Devenyi GA, Jezzard P, Hennessy TJ, Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)-An open source, MATLAB-based toolkit. Magn Reson Med. 2017 Jan;77(1):23-33. doi: 10.1002/mrm.26091. Epub 2015 Dec 30. PMID: 26715192.
[11] Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993 Dec;30(6):672-9. doi: 10.1002/mrm.1910300604. PMID: 8139448.
[12] van de Bank BL., Emir EU., Boer VO. Van Asten JJA., Maas MC, Wijnen JP., Kan HE., Oz G., Klomp DWJ., Scheenen RWJ. Multi-center reproducibility of neurochemical profiles in the human brain at 7 Tesla. NMR Biomed 2015; 18:306-316 https://doi.org/10.1002/nbm.3252
Figures