0492
Prospective motion-corrected spectroscopy in the human cervical spinal cord
Isaac M Adanyeguh1, Pierre-Gilles Henry1, and Dinesh K Deelchand1
1Center for Magnetic Resonance Imaging, University of Minnesota, Minneapolis, MN, United States
1Center for Magnetic Resonance Imaging, University of Minnesota, Minneapolis, MN, United States
Synopsis
Keywords: Spectroscopy, Spinal Cord
Proton magnetic resonance spectroscopy in the spinal cord is particularly challenging and motion remains one of biggest obstacles. Although several prospective motion correction techniques currently exist in the human brain, none have yet been reported in the spinal cord. Here we report prospective motion correction in the spinal cord using reduced-field-of-view 2DRF excitation. Results show than spectral quality were similar with and without motion. This study demonstrates the feasibility of prospective motion correction for spinal cord MRS.Introduction
Proton magnetic resonance spectroscopy (MRS) is an invaluable tool for probing metabolites concentrations in vivo. However, due to long scan times, subject motion is a major concern during MRS acquisition. Several prospective motion-correction techniques1-5 currently exist in the human brain, however, none have yet been reported in the spinal cord.MRS in the spinal cord is particularly challenging and motion remains one of biggest obstacles. Due to the small size of the spinal cord, a small motion (even a few mm) can result in significant voxel mislocalization and degraded spectral quality, especially lipid contamination from surrounding discs and vertebrae. Based on our lab experience acquiring spinal cord spectra in clinical research studies, up to half of MRS acquisitions are affected by subject motion6. This requires immediate operator intervention to reimage, reposition and reshim the voxel, thereby lengthening the total scan time. One MRS study reported real-time motion tracking with retrospective rejection of corrupted data using imaging navigators around the spinal cord as proxies for spinal cord motion7.
Therefore, the goal of the current study was to develop and implement a prospective motion-corrected MRS sequence for acquisition of high-quality spectra even in the presence of motion in the spinal cord. The proposed motion navigator is based on reduced-field-of-view (rFOV) 2DRF excitation (single-band and multi-band) to limit contamination from tissue far away from the cord and achieve higher spatial resolution.
Methods
A motion navigator was implemented based on rFOV 2DRF excitation8 with EPI readout. A 2DRF pulse (32.5ms) excited a rectangular column akin to a slice perpendicular to the cord (40 mm in Y and 5 mm in Z). The EPI readout field-of-view was 50mm in X (readout) and 100 mm in Y (phase encoding). At the start of the study, a reference volume consisting of 20 slices (matrix=64x64, TE=48ms) was acquired using 20 single-band rFOV pulses. During the single-voxel MRS scan, the navigator consisted of acquiring 2 slices (chosen to not overlap with the MRS voxel) during each TR. The latter rFOV images were acquired with a multi-band 2DRF pulse with blipped-CAIPI gradient during readout to excite both slices simultaneously9.In our experience, subject motion results in translation of the spinal cord voxel in the X-Y plane, with little rotation. Therefore, as a first approximation, motion translation in X and Y directions were estimated by registering the two navigator images with the reference volume, achieved using the discrete Fourier Transform registration10.
The sLASER sequence was modified to incorporate the real-time image-based rFOV motion navigator, as well as real-time shim and frequency corrections3-4. Figure 1 shows a schematic of the prospective motion correction algorithm.
All experiments were performed on a 3T Prisma (Siemens) scanner with a 64-channel head coil. The study was approved by institutional review board and written informed consent was obtained from the participating healthy subjects. The protocol started by acquiring a reference volume. T2 turbo spin-echo images were then acquired to position a 7x8x33 mm3 VOI in the spinal cord along C3-C5. Single-voxel sLASER data (TR/TE=5000/28ms) was obtained with water suppression achieved using metabolite cycling plus a single WS pulse for partial water saturation to limit the intensity of sidebands.Three configurations were tested: (i) without subject motion (baseline, 128 averages), (ii) with subject motion and motion-correction disabled (NoCo, 32 averages), (iii) with subject motion and motion, shim and frequency navigators enabled (shMoco, 128 averages). During the MRS measurements, the subjects were instructed to raise their chin up when prompted.
Results
Figure 2A shows the reference rFOV images (20 slices) acquired from the cervical spinal cord over the whole imaging FOV. The spinal cord is visible in almost all slices. Figure 2B shows the multi-band rFOV navigator images (2 slices around the MRS voxel) at the start of the scan, after the subject lifted their chin and the corrected image in the next TR.The mean water linewidth was 12.1±0.4 Hz in the MRS voxel using system B0 shimming routine. sLASER spectra acquired in the spinal cord from two participants are shown in Figure 3 using the 3 configurations (baseline, NoCo and shMoco). As expected lipid signal was dominant in the spectrum when the subject lifted their chin with NoCo while comparable spectral pattern was observed without subject motion (baseline) and with subject motion and prospective motion and shim correction (ShMoCo).
Discussion and Conclusion
This study shows the feasibility of correcting for motion in real-time when acquiring MRS data in the spinal cord. Correcting for translations along X and Y seems suitable for spinal cord and this results in spectra without lipid contamination. Although the spectral pattern and linewidth were comparable, the signal-to-noise was lower with shMoco compared to baseline condition. This could be related to 1) the voxel containing more CSF due to small errors in voxel localization after correction, or 2) a change in coil sensitivity when the subject lifted their chin or 3) a loss in magnetization due to presence of navigators. We will further investigate this effect. In conclusion, prospective motion-correction will be beneficial for spinal cord MRS acquisitions in both research and clinical settings without lengthening the total scan time.Acknowledgements
This work was supported by funding from the National Institutes of Health (NIH) R01 EB030000, P41 EB027061, and P30 NS076408.References
- Zaitsev M, Speck O, Hennig J, Büchert M, Single-voxel MRS with prospective motion correction and retrospective frequency correction. NMR Biomed., 2010, 23: 325-3322.
- Keating B, Deng W, Roddey JC, et al. Prospective motion correction for single-voxel 1H MR spectroscopy. Magn Reson Med. 2010; 64(3):672-6793.
- Hess AT, Tisdall MD, Andronesi OC, Meintjes EM, van der Kouwe AJW, Real-Time Motion and B0 Corrected Single Voxel Spectroscopy Using Volumetric Navigators, Magn Reson Med 2011; 66:314–3234.
- Deelchand DK, Joers JM, Auerbach EJ, Henry PG. Prospective motion and B0 shim correction for MR spectroscopy in human brain at 7T. Magn Reson Med. 2019;82(6):1984-19925.
- Jayadev NB, Henry PG, Deelchand DK, Prospective Motion Correction for MR spectroscopy in the human brain using multi-slice Spiral Navigator, Proc. Intl. Soc. Mag. Reson. Med. 2022; 31: 49946.
- Joers JM, Adanyeguh IM, Deelchand DK, Hutter DH, Eberly LE, Iltis I, Bushara KO, Lenglet C Deelchand DK, Iltis I, Hutter D, Bushara KO, Oz G, Lenglet C, Henry PG, Spinal cord magnetic resonance imaging and spectroscopy detect early-stage alterations and disease progression in Friedreich ataxia, Brain Communications, 2022, volume 4, Issue 57.
- Hock A and Henning A, Motion correction and frequency stabilization for MRS of the human spinal cord, NMR Biomed. 2016; 29: 490–4988.
- Saritas, EU, Cunningham CH, Lee JH, Han ET, Nishimura DG, DWI of the spinal cord with reduced FOV single-shot EPI. Magn Reson Med, 2008. 60(2): p. 468-4739.
- Finsterbusch J, Multi-Band-Accelerated T2*-Weighted Inner-Field-of-View EPI of the Human Spinal Cord with Slice-Specific z-Shimming, Proc. Intl. Soc. Mag. Reson. Med. 2018; 26: 544810.
- Manuel Guizar-Sicairos, Samuel T. Thurman, and James R. Fienup, Efficient subpixel image registration algorithms, Opt. Lett. 2008; 33: 156-158
Figures
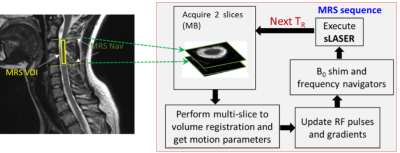
Figure 1: Overview of the prospective motion-correction in the spinal cord.
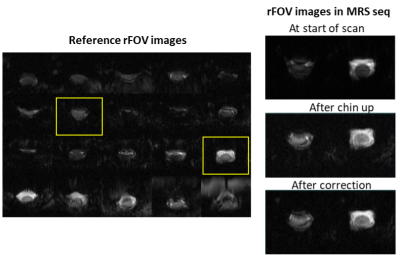
Figure 2: Gradient-echo EPI images acquired from the spinal cord in vivo using reduced FOV 2DRF excitation at 3T. A) Reference 20 images acquired using single-band 2DRF pulse and B) multi-band rFOV images acquired inside the MRS sequence at the start of the MRS scan, during the MRS scan after subject was instructed to lift their chin up and the corrected images acquired on the next TR.
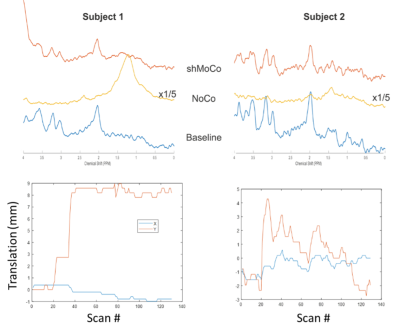
Figure 3: sLASER spectra from the C3-C5 region acquired during baseline (blue, no subject motion), NoCo (yellow, subject motion with no correction) and shMoCo (red, subject motion with prospective motion and shim correction) from 2 subjects. The corresponding translation plots measured during shMoCo are also illustrated.
DOI: https://doi.org/10.58530/2023/0492