0487
Biophysical deformation in brain-around-tumor on MRI can distinguish radionecrosis vs recurrent tumor in brain metastases: A feasibility study
Hyemin Um1, Virginia Hill2, Marwa Ismail3, Sushant Puri4, Ameya Nayate5, Prateek Prasanna6, Lisa Rogers5, Jennifer Yu7, and Pallavi Tiwari3
1Case Western Reserve University, Cleveland, OH, United States, 2Northwestern Medicine, Chicago, IL, United States, 3University of Wisconsin-Madison, Madison, WI, United States, 4Johns Hopkins Medicine, Baltimore, MD, United States, 5University Hospitals Cleveland Medical Center, Cleveland, OH, United States, 6Stony Brook University, Stony Brook, NY, United States, 7Cleveland Clinic, Cleveland, OH, United States
1Case Western Reserve University, Cleveland, OH, United States, 2Northwestern Medicine, Chicago, IL, United States, 3University of Wisconsin-Madison, Madison, WI, United States, 4Johns Hopkins Medicine, Baltimore, MD, United States, 5University Hospitals Cleveland Medical Center, Cleveland, OH, United States, 6Stony Brook University, Stony Brook, NY, United States, 7Cleveland Clinic, Cleveland, OH, United States
Synopsis
Keywords: Quantitative Imaging, Machine Learning/Artificial Intelligence
A significant challenge in the management of metastatic brain tumors following radiation therapy is distinguishing radiation necrosis from tumor recurrence. Differential diagnosis is difficult on routine MRI and patients are subject to invasive procedures to confirm the absence of disease. We explored the feasibility of deformation features from the normal parenchyma to identify disease recurrence versus radiation effects. Our results suggest that measurements of the subtle tissue deformations in the normal-appearing brain regions may elucidate differences in the tissue microarchitecture of radionecrosis and tumor recurrence and may serve as surrogate markers to non-invasively characterize treatment response in brain metastases.Introduction
A significant challenge in management of brain metastases, following standard-of-care treatment regimen, is the distinction of radionecrosis from tumor recurrence. Radionecrosis is a delayed treatment-related effect that affects roughly 20-30% of all patients1, and mimics the appearance of tumor recurrence on follow-up MRI scans. Visual inspection by trained radiologists has been reported to have a diagnostic accuracy of 50-60% at best in distinguishing the two conditions.2 In the absence of reliable non-invasive approaches, patients often need to undergo invasive interventions, including intracranial biopsies, for disease confirmation. However, intra-cranial biopsies carry a morbidity rate of 10% and a diagnostic accuracy of 85-90% due to sampling error and inter-reader variability. There is hence a pressing clinical need to reliably and non-invasively distinguish radionecrosis from tumor recurrence, to improve treatment management of brain metastases.It is suggested that radionecrosis causes a pronounced local tissue reaction with an inflammatory component, leading to a more pronounced enhancement in Edema. This, in turn, suggests that the lesion-microenvironment (LME) for radionecrosis is fundamentally different from that of tumor recurrence. Unfortunately, guidelines set by RANO/Macdonald’s criteria3,4 are solely based on 2-dimensional measurements of the enhancing tumor alone. In this work, we posit that structural displacements in the “normal” parenchyma, as reflected on follow-up Gadolinium-T1w (Gd-T1w), T2w, FLAIR scans, will be able to comprehensively characterize the “sub-visual” differences in LME in patients with radionecrosis versus those with tumor recurrence. While previous studies have explored textural radiomic features from the lesion confines to distinguish radionecrosis versus tumor recurrence, our work presents the first approach to explicitly account for measurements relating to tissue deformations from the “normal” parenchyma to distinguish the two conditions.
Methods
A total of 82 studies with post-treatment Gd-contrast T1w, T2w, and FLAIR MRI scans were retrospectively obtained from two different institutions, the Cleveland Clinic Foundation (CCF) and University Hospitals, Cleveland (UH). The CCF cohort was used for training and consisted of 61 studies, comprising 23 tumor recurrence and 38 radionecrosis cases. The UH cohort was used for holdout testing and included 21 studies, consisting of 13 recurrence and 8 radionecrosis cases. Inclusion criteria were that (a) patients received radiation therapy and demonstrated a suspicious lesion on follow-up imaging, and (b) had pathological confirmation of radionecrosis/tumor recurrence. Preprocessing involved co-registration of all MR sequences, followed by skull stripping, bias field correction and intensity standardization. Two expert readers segmented the necrotic core, enhancing tumor, and peritumoral edema using the T1w, T2w, and FLAIR scans. In order to compute deformations, processed MRIs were diffeomorphically registered to the MNI brain atlas (MNI152; Montreal Neurological Institute) using ANTs (Advanced Normalization Tools) SyN (Symmetric Normalization) toolbox.5 This included aligning the BAT region (MR volume exclusive of necrosis, enhancinand edema sub compartments) with the healthy MNI atlas, followed by inverse mapping of patient volume to MNI atlas. This mapping yielded the tissue deformation in the normal appearing BAT regions of every patient MRI. Voxel-wise tissue deformations were obtained by calculating the Euclidean norm of the absolute values of the deformation orientations. First order statistics (mean, median, standard deviation, skewness, and kurtosis) were calculated from uniformly sized (5-mm) annular bands, starting from the peritumoral edema (PE) boundaries and encompassing the entire BAT up to 60 mm from the PE (0<d<60mm where d is the distance from the PE). This resulted in 60 deformation features from 12 annular bands of the BAT region for every MR protocol. Features were selected sequentially using a Linear Discriminant Analysis (LDA) classifier in a 3-fold cross-validation with 50 iterations. The most discriminatory features were those selected at least 10% of the time in the training set, which were then evaluated on the test set to distinguish radionecrosis versus tumor recurrence. Similarly, the most discriminatory features from each protocol were used to evaluate the multi-parametric deformation model.Results and Discussion
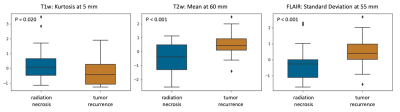
Comparative analysis of deformation features from individual MR sequences revealed that features from the BAT region in FLAIR studies were more discriminative in distinguishing radiation effects from tumor recurrence, than the other two protocols, with an accuracy of 68.1 ± 6.80% in the training set and 68.0% in the test set. The combination of features from different MR sequences improved the classifier’s accuracy to 71.8 ± 5.91% in the training set, with an accuracy of 68.0% on the test set. The 3 features that were most often selected during cross validation in the multi-parametric model were Kurtosis at 5 mm on T1w, Mean at 60 mm on T2w, and Standard Deviation at 55 mm on FLAIR. Our results suggest that: (1) deformation magnitudes seem to have extreme values near the immediate tumor confines of recurrent tumors versus lesions showing radionecrosis; and (2) subtle structural deformation changes may be pertinent all the way up to 60 mm within the BAT region.Conclusion
We presented an approach to interrogate the subtle biophysical deformations from the BAT regions in brain metastases to distinguish disease recurrence from radiation necrosis on multi-parametric MRI. Our results demonstrate the feasibility of deformation analysis, which in future, could be used in conjunction with textural radiomic analysis from the lesion confines to improve distinction of radionecrosis from tumor recurrence.Acknowledgements
No acknowledgement found.References
- Kohutek ZA, Yamada Y, Chan TA, Brennan CW, Tabar V, Gutin PH, Yang TJ, Rosenblum MK, Ballangrud Å, Young RJ, Zhang Z, Beal K. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol. 2015 Oct;125(1):149-56. doi: 10.1007/s11060-015-1881-3. Epub 2015 Aug 26. PMID: 26307446; PMCID: PMC4726630.
- Verma N, Cowperthwaite MC, Burnett MG, Markey MK. Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro Oncol. 2013 May;15(5):515-34. doi: 10.1093/neuonc/nos307. Epub 2013 Jan 16. PMID: 23325863; PMCID: PMC3635510.
- Leao DJ, Craig PG, Godoy LF, Leite CC, Policeni B. Response Assessment in Neuro-Oncology Criteria for Gliomas: Practical Approach Using Conventional and Advanced Techniques. AJNR Am J Neuroradiol. 2020 Jan;41(1):10-20. doi: 10.3174/ajnr.A6358. Epub 2019 Dec 19. PMID: 31857322; PMCID: PMC6975322.
- Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010 Apr 10;28(11):1963-72. doi: 10.1200/JCO.2009.26.3541. Epub 2010 Mar 15. PMID: 20231676.
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008 Feb;12(1):26-41. doi: 10.1016/j.media.2007.06.004. Epub 2007 Jun 23. PMID: 17659998; PMCID: PMC2276735.
DOI: https://doi.org/10.58530/2023/0487