0482
Multiscale Entropy Analysis of Resting State fMRI in Pre-Adolescents with ADHD1Mark and Mary Stevens Neuroimaging and Informatics Institute, University of Southern California, Los Angeles, CA, United States, 2Department of Psychiatry and Behavioral Sciences, University of Southern California, Los Angeles, CA, United States
Synopsis
Keywords: Data Analysis, fMRI (resting state)
The current study used resting state functional magnetic resonance imaging to measure the complexity of pre-adolescents with ADHD but no other comorbidities. We ran a factorial 2 (Group: ADHD, Control) by 2 (Sex: Male, Female) analyses of covariance on the multiscale entropy (MSE) maps with the pubertal development status as a covariate. It revealed MSE values were significantly lower in the ADHD group than the control group in various regions, including bilateral superior frontal gyrus, bilateral precuneus, right inferior/middle frontal gyrus/postcentral gyrus/insular, and left precentral gyrus/middle cingulate gyrus (t = -3.131 to -2.445, p < 0.015).Introduction
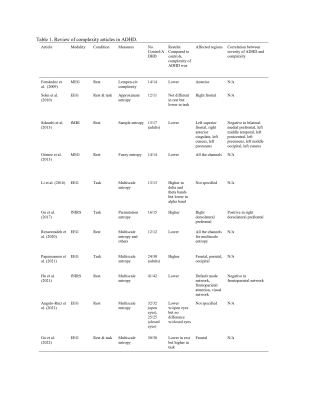
Attention deficit hyperactivity disorder (ADHD) is characterized by a persistent pattern of inattention and/or hyperactivity and impulsivity that causes impairment in functioning and development. ADHD occurs worldwide, and is estimated to affect 5% children and approximately 2.5% of adults1. In the past decade, nonlinear analyses of neural signals, such as complexity have been implemented in either healthy or diseased populations2-4. Complexity studies on ADHD are few but findings across modalities including EEG, MEG and fNIRS were generally consistent in revealing lower complexity in ADHD as compared to controls during the resting state, but higher complexity during task performance (Table 1)5-15. However, only one study reported differences in fMRI complexity12, and no study investigated fMRI complexity differences in children or pre-adolescents when ADHD is initially diagnosed. In this study we report the first study to evaluate complexity of resting state fMRI (rsfMRI) in pre-adolescents with ADHD but no other comorbidities.Method
We used baseline demographic, clinical, T1 structural, and rsfMRI data from the Adolescent Brain and Cognitive Development (ABCD) Study, the largest pediatric brain imaging study in the USA. All T1-weighted scans were acquired with voxel resolution = 1mm3, 256 × 256 matrix, flip angle = 8°, and 2x parallel imaging. The other scan parameters varied by scanner platform, i.e., Siemens Prisma, Philips, or GE 3T scanner16. Each participant had 3-4 eyes-open (passive crosshair viewing) rsfMRI scans, each of which was approximately 5-minutes in duration. All rsfMRI scans were collected using a gradient-echo EPI sequence of 383 volumes in total (voxel resolution = 2.4 mm3, 60 slices, 90 × 90 matrix, FOV = 216 × 216, TR = 800 ms, TE = 30 ms, flip angle = 52°, 6-factor MultiBand Acceleration). Head motion was monitored during scan acquisition using real-time procedures to adjust scanning procedures as necessary. The ADHD patients were selected according to the diagnosis of Kiddie-Schedule for Affective Disorders and Schizophrenia (KSADS)-parent report and each had no other psychiatric diagnoses. The final study sample included 63 ADHD subjects and 92 controls matched in terms of age (p = 0.392), sex (p = 0.381), and pubertal development status (p = 0.799, Table 2). The functional image was normalized to the standard space defined by the template T1-weighted image. Spatial preprocessing was followed by temporal preprocessing using the CONN toolbox (Conn: fMRI functional connectivity toolbox). Multiscale entropy (MSE) was computed using the in-house developed LOFT Complexity Toolbox (github.com/kayjann/complexity). In the MSE calculation, we set the pattern length m = 2, the sensitivity threshold r = 0.3, and the number of temporal scales to 15. We ran a factorial 2 (Group: ADHD, Control) by 2 (Sex: Male, Female) analysis of covariance (ANCOVA) on the MSE maps with pubertal development status as a covariate.Results
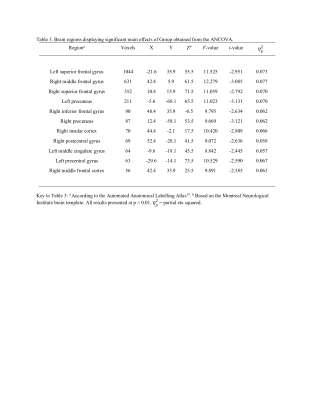
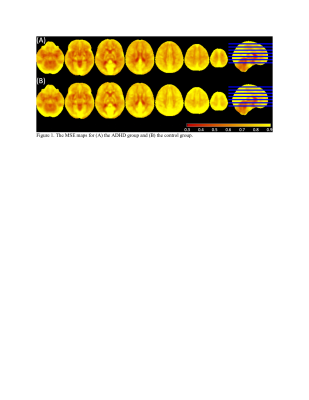
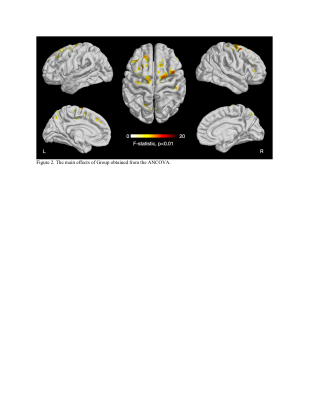
The average MSE maps of the ADHD group and the control group are displayed in Figure 1. The ANCOVA revealed significant main effects of Group (F = 8.842 to 12.279, p < 0.01, partial eta squared = 0.057 to 0.077, corrected for multiple comparison using cluster size threshold estimation > 50, Figure 2, Table 3). The post-hoc test demonstrated widespread significantly lower MSE in the ADHD group than the controls in multiple regions, including bilateral superior frontal gyrus, bilateral precuneus, right middle frontal gyrus, right inferior frontal gyrus, right insular cortex, left precentral gyrus, left middle cingulate gyrus, and right postcentral gyrus (t = -3.131 to -2.445, p < 0.015, Table 3). There were no areas with significantly increased MSE in ADHD. We further tested whether there is an association between MSE and the CBCL DSM5 ADHD score but correlation analysis with and without accounting for sex and pubertal development status did not reach significance (p < 0.01, number of voxels > 50).Conclusion
The ADHD group was characterized by reduced frontal cortex rsfMRI complexity as compared to the matched controls. Specifically middle and superior frontal gyri representing the dorsolateral prefrontal cortex as well as cingulate cortex were affected. The observation of reduced entropy is consistent with existing literature using other neuroimaging modalities and also with hypotheses of altered function of inhibitory control networks in ADHD.Acknowledgements
This project was funded by NIH 1R01AG066711 (Jann/Wang).References
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. Jun 2007;164(6):942-8. doi:10.1176/ajp.2007.164.6.9422.
Sun J, Wang B, Niu Y, et al. Complexity Analysis of EEG, MEG, and fMRI in Mild Cognitive Impairment and Alzheimer's Disease: A Review. Entropy (Basel). Feb 20 2020;22(2)doi:10.3390/e220202393.
Takahashi T. Complexity of spontaneous brain activity in mental disorders. Prog Neuropsychopharmacol Biol Psychiatry. Aug 01 2013;45:258-66. doi:10.1016/j.pnpbp.2012.05.0014.
Fernández A, Gómez C, Hornero R, López-Ibor JJ. Complexity and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. Aug 01 2013;45:267-76. doi:10.1016/j.pnpbp.2012.03.0155.
Fernández A, Quintero J, Hornero R, et al. Complexity analysis of spontaneous brain activity in attention-deficit/hyperactivity disorder: diagnostic implications. Biol Psychiatry. Apr 01 2009;65(7):571-7. doi:10.1016/j.biopsych.2008.10.0466.
Gómez C, Poza J, Fernández A, Bachiller A, Gómez J, Hornero R. Entropy analysis of MEG background activity in attention-deficit/hyperactivity disorder. Annu Int Conf IEEE Eng Med Biol Soc. 2013;2013:5057-60. doi:10.1109/EMBC.2013.66106857.
Hu Z, Liu L, Wang M, et al. Disrupted signal variability of spontaneous neural activity in children with attention-deficit/hyperactivity disorder. Biomed Opt Express. May 01 2021;12(5):3037-3049. doi:10.1364/BOE.4189218.
Chenxi L, Chen Y, Li Y, Wang J, Liu T. Complexity analysis of brain activity in attention-deficit/hyperactivity disorder: A multiscale entropy analysis. Brain Res Bull. 06 2016;124:12-20. doi:10.1016/j.brainresbull.2016.03.0079.
Papaioannou AG, Kalantzi E, Papageorgiou CC, et al. Complexity analysis of the brain activity in Autism Spectrum Disorder (ASD) and Attention Deficit Hyperactivity Disorder (ADHD) due to cognitive loads/demands induced by Aristotle's type of syllogism/reasoning. A Power Spectral Density and multiscale entropy (MSE) analysis. Heliyon. Sep 2021;7(9):e07984. doi:10.1016/j.heliyon.2021.e0798410.
Rezaeezadeh M, Shamekhi S, Shamsi M. Attention Deficit Hyperactivity Disorder Diagnosis using non-linear univariate and multivariate EEG measurements: a preliminary study. Phys Eng Sci Med. Jun 2020;43(2):577-592. doi:10.1007/s13246-020-00858-311.
Sohn H, Kim I, Lee W, et al. Linear and non-linear EEG analysis of adolescents with attention-deficit/hyperactivity disorder during a cognitive task. Clin Neurophysiol. Nov 2010;121(11):1863-70. doi:10.1016/j.clinph.2010.04.00712.
Sokunbi MO, Fung W, Sawlani V, Choppin S, Linden DE, Thome J. Resting state fMRI entropy probes complexity of brain activity in adults with ADHD. Psychiatry Res. Dec 30 2013;214(3):341-8. doi:10.1016/j.pscychresns.2013.10.00113.
Angulo-Ruiz BY, Muñoz V, Rodríguez-Martínez EI, Cabello-Navarro C, Gómez CM. Multiscale entropy of ADHD children during resting state condition. Cognitive Neurodynamics. 2022;doi:10.1007/s11571-022-09869-014.
Gu C, Liu ZX, Woltering S. Electroencephalography complexity in resting and task states in adults with attention-deficit/hyperactivity disorder. Brain Commun. 2022;4(2):fcac054. doi:10.1093/braincomms/fcac05415.
Gu Y, Miao S, Han J, et al. Complexity analysis of fNIRS signals in ADHD children during working memory task. Sci Rep. 04 11 2017;7(1):829. doi:10.1038/s41598-017-00965-416.
Casey BJ, Cannonier T, Conley MI, et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci. 08 2018;32:43-54. doi:10.1016/j.dcn.2018.03.00117.
Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc. Apr 1988;17(2):117-33. doi:10.1007/BF0153796218.
Achenback TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: ASEBA; 2001.19.
Rolls ET, Huang CC, Lin CP, Feng J, Joliot M. Automated anatomical labelling atlas 3. Neuroimage. 02 01 2020;206:116189. doi:10.1016/j.neuroimage.2019.116189
Figures