0481
fMRI with whole-brain coverage, 75ms temporal resolution and high SNR by combining HiHi reshuffling and Multiband imaging1Laboratory for Social and Neural Systems Research (SNS Lab), University of Zürich, Zürich, Switzerland, 2Institute for Biomedical Engineering, ETH Zürich and University of Zürich, Zürich, Switzerland, 3Norwegian University of Science and Technology, Trondheim, Norway, 4Siemens Healthineers International AG, Zürich, Switzerland, 5Swiss Center for Musculoskeletal Imaging (SCMI), Balgrist Campus, Zürich, Switzerland, 6Advanced Clinical Imaging Technology (ACIT), Siemens Healthineers International AG, Lausanne, Switzerland
Synopsis
Keywords: Data Processing, fMRI
By combining an fMRI data shuffling method and multiband accelerated acquisition, we were able to measure the hemodynamic response in the primary motor and visual cortices with 75ms temporal resolution while maintaining high SNR. With the addition of multiband imaging, we were able to achieve whole-brain coverage in a feasible scan time, and use appropriate event-related stimulus paradigm to map the BOLD response in the primary motor and visual cortices in a combined experiment.Introduction
Functional magnetic resonance imaging (fMRI) exploits the different magnetic properties of arterial and venous blood1 to measure the blood oxygen level dependent (BOLD) signal for imaging neuronal activity2. In addition to standard EPI imaging3, there are methods to increase the temporal resolution of fMRI data4;5, but these are associated with a reduction in MRI signal due to incomplete T1 relaxation and thus a lower signal-to-noise ratio (SNR). In this study, we combined a recently published method for data reordering6 (HiHi fMRI) with Multiband (MB)7;8 acquisition on a 7T MRI scanner to acquire whole-brain measurements of the BOLD response with high temporal resolution (75ms) and high SNR. Visual and motor stimuli were used to induce a BOLD response in the primary motor and visual cortices.Methods
Motor and visual taskThe visual stimuli, which also served as the cues for performing the motor task, were programmed in nordicAktiva (Nordic NeuroLab, Bergen, Norway) and shown on their MRI-compatible screen (40” in diameter with 3,840 × 2,160 pixels). Participants viewed a uniform gray background (R/G/B-127/127/127) and were asked to fixate on a small white cross. The visual stimulus was a black and white checkerboard that flickered at 8 Hz for 375 ms, prompting the participants to press a response grip (Nordic NeuroLab) once with the index finger and thumb of their dominant hand (motor response).
Data acquisition
Participants
The experiments involved 2 adult participants (1 right-handed male and 1 left-handed female), with the approval of the local ethics committee (2020-00208_TS12) and after signing a written informed consent. One of the participant was scanned on two different days to assess test-retest reproducibility.
Imaging protocol
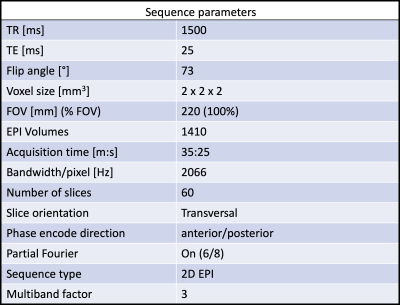
All MRI data were collected on a 7T MAGNETOM Terra scanner (Siemens Healthcare, Erlangen, Germany) and a single-channel transmit and 32ch receive head coil (Nova Medical Inc., MA, United States). The visual and motor experiments were performed in the same fMRI scan of approximately 35 minutes. Each session allowed for 100 visual stimuli (and concurrent motor tasks) but in a pseudo-random order only 80 of these were given. A baseline signal was extracted from the 20 time-points without visual stimulus or motor response. We utilized a 2D EPI3 sequence combined with multiband acceleration factor of 37;8. Relevant sequence acquisition parameters are listed in Table 1. Assuming a BOLD response lasting 20 seconds, 14 EPI volumes of 60 slices were collected after each stimulus. At the beginning of each experiment, 5 dummy scans were collected, resulting in a total of 1410 EPI-volumes.
Data analysis
The data analysis was performed with custom-made Matlab scripts and SPM129. First, each time-series was aligned to the first EPI volume using rigid body correction and subsequently the voxel-wise signal evolution was detrended using a second-degree polynomial regression. Second, we reshuffled the original time-series into three new pseudo time-series using either a) the timing of the visual stimulus, b) the slightly delayed timing of the respective motor response (HiHi fMRI6) or c) the timing when the visual stimulus would have been shown but randomly omitted (for the reference baseline time-series). After restructuring the data into the HiHi time-series, we searched for BOLD responses in the primary visual and motor cortices and compared them with the respective pseudo-time series of the control experiment. In a second analysis, we compared the BOLD responses of the two participants in both cortical areas, scaled with respect to their individual temporal mean of the baseline experiment.
Results
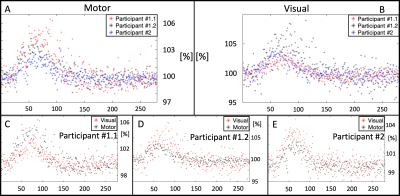
The top row (green dots) in Fig. 1 show the time-course of a BOLD response from a single voxel in both the motor and visual cortices at a temporal resolution of 75ms, whereas the bottom row (blue dots) show the time-course of the corresponding voxels of the control experiment (i.e. without visual stimulus or motor response). Although rare, in the second session Participant 1 did not respond at least once in each slice, resulting in missing data points in the HiHi time series, as shown in Figure 1B, top left. Oscillatory artifacts occur to varying degrees in the hemodynamic response and the baseline at intervals of about 1TR (= 20 slices) (cf. Fig. 1B, top and bottom left), which may be caused by inadequate clean up of the head motion or slow signal drift in the time-series data. Fig. 2 shows the time-course of all participants in both cortices.Discussion and conclusion
As a proof-of-principle, we provided empirical evidence that the combination of both MB and HiHi fMRI, can reveal BOLD responses in the visual and motor cortex with high temporal resolution (75ms) and high SNR, offering further possibilities for capturing fast fMRI dynamics while simultaneously covering larger brain areas and optimizing scan time. In future work, correcting for undesired physiological signal components or scanner-related signal drifts will need to be addressed. Such corrections will likely clean up the oscillatory behavior of the re-shuffled HiHi fMRI data (see Fig. 1).Acknowledgements
We thank Dr. Gergely David for his assistance with recruiting and booking the participants for this study.References
1. Linus Pauling and Charles D Coryell. The magnetic properties and structure of hemoglobin, ox yhemoglobin and carbonmonox yhemoglobin, 1936.
2. Pierre J Magistretti and Luc Pellerin. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging.
3 . Franz Schmitt, Michael K. Stehling, and Robert Turner. Echo-Planar Imaging. Springer Berlin Heidelberg, 1998.
4. David A. Feinberg and Essa Yacoub. The rapid development of high speed, resolution and precision in fmri, 8 2012.
5. Nick Todd, Steen Moeller, Edward J. Auerbach, Essa Yacoub, Guillaume Flandin, and Nikolaus Weiskopf. Evaluation of 2d multiband epi imaging for high-resolution, whole-brain, task-based fmri studies at 3t: Sensitivity and slice leakage artifacts. NeuroImage, 124:32–42, 1 2016.
6. Zoltan Nagy, Chloe Hutton, Gergely David, Natalie Hinterholzer, Ralf Deichmann, Nikolaus Weiskopf, and S Johanna Vannesjo. HiHi fMRI: a data-reordering method for measuring the hemodynamic response of the brain with high temporal resolution and high SNR. Cerebral Cortex, 09 2022. bhac364.
7. David J Larkman, Joseph V Hajnal, Amy H Herlihy, Glyn A Coutts, Ian R Young, and G¨o Sta Ehnholm. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited, 2001.
8. Steen Moeller, Essa Yacoub, Cheryl A. Olman, Edward Auerbach, John Strupp, Noam Harel, and Kˆamil Uˇgurbil. Multiband multislice ge-epi at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fmri. Magnetic Resonance in Medicine, 63:1144–1153, 2010.
9. K.J. Friston, J. Ashburner, S.J. Kiebel, T.E. Nichols, and W.D. Penny, editors. Statistical Parametric Mapping:The Analysis of Functional Brain Images. Academic Press, 2007.
Figures