0472
Phase-Based Registration for Visualisation of Pulsatile Brain Motion1Auckland Bioengineering Institute, University of Auckland, Auckland, New Zealand, 2Department of Engineering Science, University of Auckland, Auckland, New Zealand, 3Department of Anatomy and Medical Imaging & Center for Brain Research, University of Auckland, Auckland, New Zealand, 4Mātai Medical Research Institute, Tairāwhiti-Gisborne, New Zealand
Synopsis
Keywords: Data Processing, Brain, Chiari 1 Malformation
Pulsatile brain motion is a valuable tool in understanding injury and congenital disorders. These pulsatile motions have a typical magnitude of < 1 pixel. Amplified MRI (aMRI) amplifies the pulsatile motion to the point of visibility, enabling qualitative examination. However, the amplification in frequency space results in nonlinear distortion of motion preventing the recovery of unaugmented displacements. Here, we propose using phase-based subpixel registration. This methodology enables the recovery of displacement quantification of pulsatile brain motion.Introduction
Chiari I malformation (CM-I) is a condition of the brain in which the cerebellar tonsils herniate into the brainstem, resulting in altered pulsatile motion throughout the brain with each cardiac cycle1. This pulsatile motion is typically smaller than a pixel. aMRI has recently been used to study this condition, as the method is able to amplify subvoxel motion.While aMRI permits qualitative determination of the condition2, the amplification performed in frequency space complicates the quantitative analysis of the resulting shifts. Instead, we propose the use of phase-based registration. Typical registration methodologies, such as normalised cross correlation, recover displacements to approximately pixel precision. Phase-based registration is a correlation approach used in image processing to estimate the relative motion between two similar images. The implemented methodology has been validated to recover shifts of less than 50 millipixels. Results are presented on both a healthy and CM-I individual using standard cardiac gated (cine) MRI data, with the ability to distinguish between the cases examined.
Methods
Image acquisition:Under ethical approval, images were acquired on a healthy volunteer and CM-I patient. The scan was performed using a 3T-Tesla (3T) GE Medical Systems SIGNA Premier system (GE, USA), SuperG Gradients (80 mT/m at 200 T/m/s) and a 48-channel head coil. A cardiac gated (‘cine’) 3D balanced steady-state free precession (bSSFP) sequence with peripheral cardiac gating was used and retrospective binning to 20 cardiac phases. The parameters were as follows: FOV = 23 cm, matrix size = 194 × 194, TR/TE/flip angle = 46 ms/1.7 ms/26 °, acceleration factor (using k-t-blast) = 8, partition thickness of 1.2 mm, resolution of 1.2 mm isotropic, scan time of 2:30 minutes for whole brain coverage.
Registration:
To recover the motion between subsequent frames, phase-based registration was used. The proposed registration methodology is based on the shift property of the Fourier transform.
For some signal f(x):
$$\mathcal{F}(f(x-𝜌)) = \mathcal{F}(f(x)) e^{2i𝜌𝜔}$$
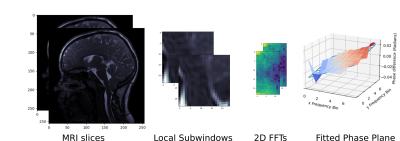
where 𝜌 is the shift, and 𝜔 is the affected frequency. As a result, if one image is a pure subpixel shift of another, the result of the normalised cross power spectrum (NCPSD) of the two images is a phase difference linearly dependent on frequency and the applied shift 3,4. A visualisation of the proposed pipeline is shown in Figure 1.
The registration presented recovers the displacement field over the central sagittal slice throughout the Cine MRI images. This slice enables identification of the cerebellar tonsils. The registration algorithm recovers shifts at distinct subwindows over the acquired input images. The presented results used 24 x 24 pixel subwindows, which were were separated with a stride of 4 pixels. The registration is run with reference to the first frame of the Cine MRI, producing absolute measurements of pixel displacement in each of the subsequent frames. The pixel displacements of the registration algorithm were converted to a displacements in millimetres, allowing the depiction of the oscillation of the cerebellar tonsils during the cardiac cycle.
Results and Discussion
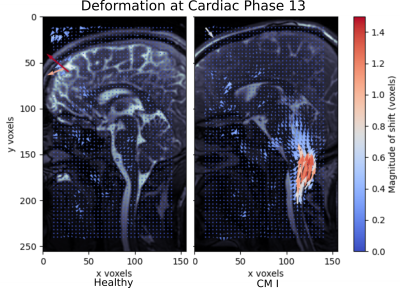
Figure 2 shows that the phase-based registration was able to highlight elevated cerebellar tonsil and brainstem motion in the CM-I patient. Here, the velocity field of the 13th cardiac phase is shown, whereby the superior/inferior motion seen closely matched the observed pattern of pulsatile brain motion in the cine MRI data. The magnitude of the motion peaked around the 13th cardiac phase. The reported displacements suggest that the patient with CM-I experienced a maximum within-plane displacement of 1.5 mm in the cerebellar tonsils, with a greater region experiencing a 0.5 mm shift. In the healthy volunteer there is no discernible motion in the cerebellar tonsil and brainstem. In both cases, we observed a noise floor of approximately 0.2 pixels or 0.25 mm over the registered region of interest.Conclusion
This study presents a new phase-based processing approach for the recovery of pulsatile motion in the brain. We have demonstrated that the magnitude of the observed mid-brain deformation agrees with the qualitative measurements seen in the literature and recovered quantitative measurements. This technique may enable the use of measured shifts to inform further understanding of both the treatment of Chiari I malformation and the understanding of the mechanics of the brain in disease and health.Acknowledgements
We gratefully acknowledge the support of the University of Auckland Foundation.References
1. Leung V, Magnussen JS, Stoodley MA, Bilston LE. Cerebellar and hindbrain motion in Chiari malformation with and without syringomyelia. J Neurosurg Spine. 2016;24(4):546-555. doi:10.3171/2015.8.SPINE153252.
2. Terem I, Ni WW, Goubran M, et al. Revealing sub-voxel motions of brain tissue using phase-based amplified MRI (aMRI). Magn Reson Med. 2018;80(6):2549-2559. doi:10.1002/mrm.272363.
3. HajiRassouliha A, Tang EJLP, Taberner AJ, Nash MP, Nielsen PMF. A Method for Three-Dimensional Measurements Using Widely Angled Stereoscopic Cameras. In: 2019 IEEE International Instrumentation and Measurement Technology Conference (I2MTC). ; 2019:1-5. doi:10.1109/I2MTC.2019.88270864.
4. Stone HS, Orchard MT, Ee-Chien Chang, Martucci SA. A fast direct Fourier-based algorithm for subpixel registration of images. IEEE Trans Geosci Remote Sens. 2001;39(10):2235-2243. doi:10.1109/36.957286
Figures