0470
Mouse sleep fMRI with simultaneous electrophysiology at 9.4T1Institute of Neuroscience, CAS Key Laboratory of Primate Neurobiology, Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China, Shanghai, China, 2Department of Anesthesia, Zhongshan Hospital, Fudan University, Shanghai, China, Shanghai, China, 3School of Biomedical Engineering, Southern Medical University, Guangzhou, China, Shanghai, China
Synopsis
Keywords: Data Analysis, Brain
Simultaneous electrophysiology and fMRI could provide both macroscopic and microscopic observations, but it is highly technically challenging and not widely used in sleep research. We developed mouse sleep fMRI based on simultaneous electrophysiology at 9.4T and allowed manifestation of the full sleep cycle (NREM/REM) during fMRI. The results revealed global state-dependent patterns. Rich state transition epochs demonstrated that state transitions were global, irreversible and sequential phenomenon, which can be predicted using LSTM RNN models. Importantly, simultaneous hippocampal recording revealed enhanced sharp-wave ripple triggered global patterns during NREM than awake state, which may attribute to co-occurrence of spindle events.
Introduction
Sleep is a universal and vital phenomenon for all species that have been carefully studied so far, but its functions and mechanisms remain to be fully understood. In the past several decades, neuronal and circuitry level microscopic studies in mice and macroscopic imaging studies in humans both provided important insight of sleep at different spatiotemporal levels1,2. However, it remains challenging to provide and integrate both local and global functional information of sleep and particularly sleep transition dynamics. We developed mouse sleep fMRI method based on simultaneous electrophysiology and achieved the recording of whole awake-sleep (NREM/REM) cycle during fMRI. Importantly, using MRI compatible graphene fiber electrodes, hippocampal LFP signal were recorded and characteristic events such as spindle and sharp-wave ripple (SWRs) were extracted during sleep fMRI.Methods
24 Male wide type C57BL/6J mice were used in the study. All MRI data were acquired with a Bruker BioSpec 9.4T scanner. An 86 mm volume coil was used for transmission and a single loop mouse head coil (Bruker, 1 cm diameter) was used for receiving. A T2 weighted RARE anatomical image (TR: 3200 ms; TE: 34 ms; matrix size: 256×128; FOV: 18×9 mm2; slice thickness: 400 μm; resolution: 70×70 μm2) was acquired for coregistration. Functional images were acquired using single-shot echo planar imaging with the following parameters: TR 2000 ms, TE 14 ms, FA 70°, matrix size 90×45 μm2 , nominal in-plane resolution 200×200 μm2, slice thickness 400 μm, slices number 22, 7200 EPI volumes. Neurophysiological signals were recorded at a sampling rate of 24414 Hz, high-pass filtered at 0.1 Hz and notch filtered at 50/100-150 Hz, except for LFP signals, which were recorded without notch filter.The correction of MRI gradient artifacts in the electrophysiological signals was conducted through FMRIB in EEGLAB (FASTR)3. The preprocessing steps of BOLD signal includes: slice-timing, realignment, registration, smooth and nuisance signal regression. Nuisance signals contain 6 head motion parameters, their 1st order first derivatives, and the first 40 principal components from the non-brain tissue signals4.
Results
Two types of MRI compatible electrodes were developed. First, a 18-contacts MRI compatible surface electrode were custom designed and manufactured for recording electrocorticogram and electromyography signals (Fig. 1a). Second, a MRI compatible depth electrode was developed with 1 electrocorticogram site, 2 electromyography sites and 1 LFP site (graphene fiber electrode) (Fig. 1b).A total of 184 hours simultaneous ECoG/LFP-fMRI recordings were acquired from 24 mice. Sleep states were conventionally classified using electrophysiological signals (Fig. 1f-g). Using the conventional GLM analysis, we mapped the brain-wide BOLD activations of NREM and REM sleep, relative to the AW state (Fig 2). In addition to static state-dependent features, the dynamics of sleep architectures have also been reported5. We conducted group PCA to BOLD signals for dimensional reduction and obtained top 100 low-dimensional patterns. Each PC exhibited non-stationary temporal weights from the start to the end of each state. Importantly, the temporal weights of PCs also exhibited diverse characteristics across state transitions, suggesting dynamic involvements of different functional networks across brain states (Fig. 3).
The dynamic characteristics of PCs across state transitions promoted us to investigate whether such transitions could be predicted by BOLD signals prior to electrophysiology defined transition time. Thus, we established a LSTM RNN model for state transition prediction, with BOLD signals preceding state transitions as model input and the brain state after such transition as model output. Results of the LSTM RNN model revealed the mean discriminate time of state transitions and several regions significantly affected the prediction of state transitions (Fig. 4).
Using the neural-event-triggered fMRI, we observed higher whole brain activations of SWR evoked BOLD signal in NREM state than AW state, suggesting state dependency of SWR evoked global patterns. SWRs exhibited a strong co-occurrence with spindle events6,7. Thus, we revealed that the spatiotemporal pattern of spindle-uncoupled SWRs in NREM state resembled the pattern of SWRs in AW state. And spindle-coupled SWRs exhibited similar spatiotemporal pattern but higher cortical activations, compared to the spindle-uncoupled SWRs. These results suggested that the co-occurrence of spindle contributed to the enhanced SWRs evoked BOLD responses in NREM state (Fig. 5).
Discussion
Our data includes abundant NREM/REM and state transition epochs, which enabled us to study the dynamics of sleep. Our results provided a global view of sleep state-dependent activations and state-transition dynamics. The preceding discriminate time and several sensitivity regions provided several information to sleep research. Furthermore, we revealed the potentiated SWRs-evoked BOLD response in NREM state compared to that in AW state, largely attributed to the coupling of spindle events.Conclusion
We developed the mouse sleep fMRI method in un-anesthetized mice based on highly MR-compatible simultaneous electrophysiology at 9.4T. For sleep research, this method provides an avenue to investigate sleep dynamics at a global scale, and has great potential to integrate local and global view of sleep when further combined with other circuitry level tools in mice. For fMRI research, our method provides a convenient platform to examine the neural basis of the arousal related fMRI signal.Acknowledgements
The authors thank the Mouse Animal Facility of CEBSIT for animal care. This work was supported by the National Natural Science Foundation of China (8217070761), Science and Technology Innovation 2030- Major Project for Brain Science and brain-like Program (2021ZD0202200).
References
1. Liu D, Dan Y. A Motor Theory of Sleep-Wake Control: Arousal-Action Circuit. Annu Rev Neurosci. 2019 Jul 8;42:27-46.
2. Tagliazucchi E, von Wegner F, Morzelewski A, Brodbeck V, Jahnke K, Laufs H. Breakdown of long-range temporal dependence in default mode and attention networks during deep sleep. Proc Natl Acad Sci U S A. 2013 Sep 17;110(38):15419-24.
3. Allen PJ, Josephs O, Turner R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage. 2000 Aug;12(2):230-9.
4. Chen X, Tong C, Han Z, Zhang K, Bo B, Feng Y, Liang Z. Sensory evoked fMRI paradigms in awake mice. Neuroimage. 2020 Jan 1;204:116242.
5. Fultz, N. E. et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 366, 628-631
6. Oyanedel CN, Durán E, Niethard N, Inostroza M, Born J. Temporal associations between sleep slow oscillations, spindles and ripples. Eur J Neurosci. 2020 Dec;52(12):4762-4778.
7. Buzsaki, G. Hippocampal sharp waves: their origin and significance. Brain Res 398, 242-252
Figures
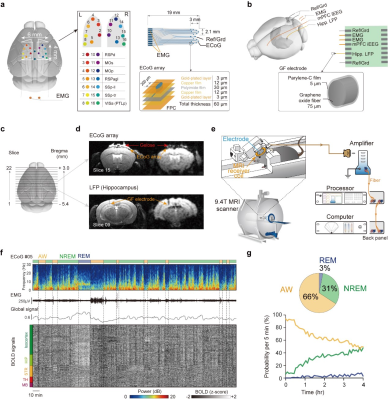
Figure 1.
Mouse sleep fMRI using MRI compatible electrophysiology recording in un-anesthetized mice.
a, b: Design and location of MRI compatible ECoG array and depth electrode. c, d: Illustration of field of view and minimal MRI artifacts. e. Schematic illustration of the mouse sleep fMRI setup. f: A representative session of mouse sleep fMRI with AW, NREM and REM sleep states. g: Averaged and time dependent probability of each brain state.
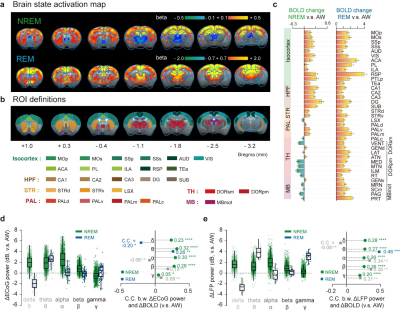
Figure 2.
Brain-wide BOLD activations of NREM and REM sleep and their electrophysiological correlates.
a: Group BOLD activation maps of NREM and REM compared to AW state. b Region-of-interest definitions. c Mean relative BOLD changes of NREM and REM compared to AW state. d-e Electrophysiological correlates of sleep dependent BOLD changes.
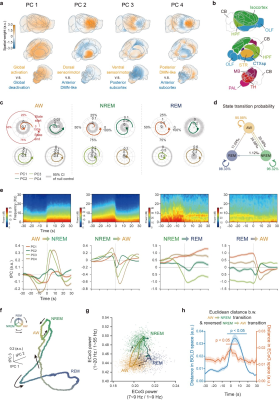
Figure 3.
Low dimensional dynamic signature across brain states.
a Spatial map for the first four PCs of BOLD signals. b Major brain divisions c Circular distribution of temporal weights of PCs of each brain state. d Transition probability of states. e Averaged electrophysiological power spectrogram and mean tPCs of BOLD signals relative to state transitions f-g Low dimensional representation of BOLD and electrophysiological signals. h Asymmetric trajectories of state transitions.
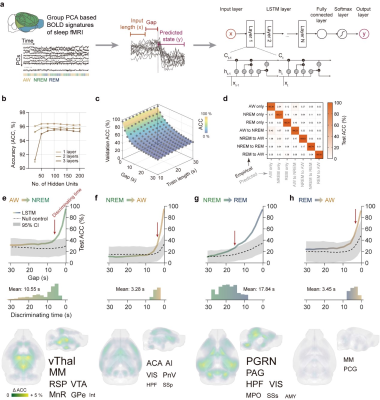
Figure 4.
LSTM RNN Prediction of state transitions based on large-scale BOLD signatures.
a Computational pipeline of the LSTM RNN model for state transition prediction. b Mean prediction accuracy of the model on the validation dataset. c Prediction accuracy on the validation dataset was primarily related to the gap times. d High prediction accuracy on the testing dataset e-h Gap time dependent test accuracy and regional sensitivity on state predictions.
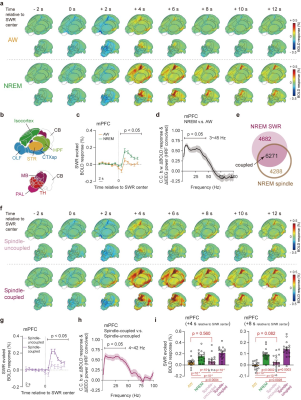
Figure 5.
State dependent global spatiotemporal patterns of SWR in AW and NREM states using neural-event-triggered fMRI
a. SWR triggered BOLD responses in AW and NREM states. b.Major brain divisions. c-d Differences of BOLD responses in mPFC between AW and NREM states and its electrophysiological correlates. e Overlap of SWR and spindle events in NREM state. f Spindle-uncoupled and coupled SWR triggered BOLD responses in NREM state. g-h Differeces of BOLD responses in mPFC between spindle coupled and uncoupled states. i BOLD responses in mPFC.