0467
Brain white matter alterations in military service members after a remote mild traumatic brain injury1National Intrepid Center of Excellence,Walter Reed National Military Medical Center, Bethesda, MD, United States
Synopsis
Keywords: Traumatic brain injury, Traumatic brain injury
In this study, we applied non-Gaussian diffusion MRI, including bi-tensor diffusion tensor imaging, diffusion kurtosis imaging, neurite orientation dispersion and density imaging and fixel-based analysis, to assess white matter disruption after a remote brain injury. The findings of both increased and decreased orientation distribution index over the forceps major and forceps minor of the corpus callous, along with increased fiber density and fiber cross-sectional area of fiber bundle over the anterior frontal region suggests a mixed pattern of white matter alterations, e.g. loosely white matter such as gliosis along with neuroplasticity and brain repair after a remote mild traumatic brain injury.Introduction
Mild traumatic brain injury (mTBI) is difficult to diagnose and characterize. Identifying underlying aberrant white matter (WM) structural changes associated with persistent post-concussive symptoms can differentiate mTBI from purely psychological disorders. Diffusion tensor imaging (DTI), assuming Gaussian diffusion within a single microstructural compartment, has been shown to be sensitive in detecting white matter (WM) microstructural changes after TBI1, but inconsistent findings among studies. DTI, not able to resolve WM fiber crossings, may be suboptimal for use in detecting subtle WM changes in mTBI due to the heterogeneity of injury mechanisms and complexity of brain tissue. Non-Gaussian diffusion-weighted MRI (dMRI) techniques such as neurite orientation dispersion and density imaging (NODDI) that relates dMRI signal to tissue features via biophysically modeling; fixel-based analysis (FBA) method that account for crossing WM fibers and can provide indices of both WM micro- and macro-structure, which have been shown to be more sensitive than DTI in investigating WM changes in mTBI2. The aim of this study is to apply non-Gaussian dMRI to investigate WM integrity in service members (SMs) after a remote brain injury.Methods
Two hundred and eleven (211) male SMs (age: 40.18 ± 5.88 years old) were selected from a larger sample of TBI cohort at the National Intrepid Center of Excellence, WRNMMC. Diagnosis of TBI during enrollment occurred via a multi-layered structured interview process screening for every potential concussive event (PCE) during military deployments and across the entire lifetime, including childhood, using a modification of the Ohio State University TBI Identification (OSU TBI-ID) instrument3. mTBI was diagnosed as previously described4. Forty-three (43) male non-TBI controls were recruited for comparison (age: 35.50 ± 8.64 years old). Simultaneous multi-slice (SMS) multi-shell dMRI was acquired using a 3T scanner equipped with a 32-channel head coil with three shells (b=1000, 2000, 3000, 1.7 mm3) and an SMS acceleration factor of three. dMRI was preprocessed with noise reduction, motion eddy current correction, and non-linear registration to the structural T2W image using the TORTOISE package. NODDI-Watson model5 was fitted to reconstruct maps of neurite density index (NDI), the intracellular volume fraction which primarily represents axonal density within WM; the volume fraction of the isotropic diffusion compartment (FISO) representing the free water content within the tissue; and orientation dispersion index (ODI) of neurites. In addition, bi-tensor free water DTI (FWDTI)6 reconstructed maps of FW-corrected DTI metrics, and diffusion kurtosis imaging (DKI)7 for reconstructing maps of radial kurtosis (RK), axial kurtosis (AK) and mean kurtosis (MK). To perform FBA, fiber orientation distributions (FOD) were estimated using the multi-shell multi-tissue constrained spherical deconvolution, and a group response function was calculated by averaging all individual response functions. A study specific, unbiased FOD template was generated from a subset of participants using linear and non-linear registration of the FOD images followed by registering all FOD images to this template.For comparing difference of neuroimaging metrics, participants were further selected in order to match their age, a cohort of 55 age-matched mTBI subjects and 55 controls between 20 and 60 years old; and another of cohort of 211 mTBI subjects and 43 controls who were in the age range between 30 years and 50 years old. General linear mixed modeling was applied to evaluate the difference of fixed effect of group difference between controls and mTBI subjects; and the association between neuroimaging metrics, e.g. non-Gaussian dMRI metrics and FBA metrics (including fiber density (FD), fiber bundle cross-section (FC), and a combined measure of fiber density and bundle cross-section (FDC)) within the mTBI group by modeling age covariate. Significance was tested with 0.05 family-wise error for correcting multiple comparisons.Results
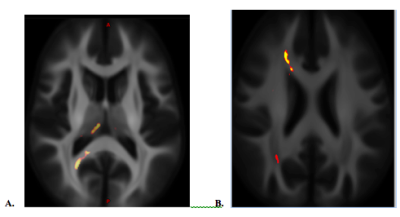
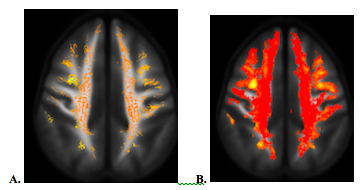
The statistical significances of group comparisons of diffusion metrics were similar between two different age-matched cohorts. Compared to the controls, mTBI subjects had higher AK, MK and ODI, but lower FISO over the dorsomedial part of the thalamus (Fig. 2A), as well as lower ODI over the paths of forceps major and forceps minor of the right corpus callosum (CC) and the anterior frontal WM fiber (Fig. 2B). For FBA, mTBI had higher FD and FDC over the anterior frontal WM (Fig. 3), and increased log(FC) over the bilateral cerebellar WM. There was no significant group difference among DTI metrics.Discussion / Conclusions
No group difference was found in DTI metrics, but WM alterations were revealed using DKI, NODDI and FBA. This suggests higher orders dMRI should be considered as a choice of option in assessing WM disruption after a remote brain injury. Besides the anterior frontal WM, the findings of WM microstructural changes in mTBI over the dorso-medial thalamus and the path of the forceps minor of the CC revealed by NODDI were consistent with the previous report2. Higher ODI and diffusion kurtosis in mTBI suggests loosely organized WM after brain injury. However, lower ODI over both of the anterior and posterior cerebral WM in the same hemisphere might be due to the coup-countrecoup mechanism (Fig. 2B). Increased FD (increased axonal density, i.e. microstructural changes) and FC (the cross-sectional area that is occupied by a fiber bundle, i.e. macrostructural changes) suggest neuroplasticity after injury (Fig. 3).Acknowledgements
Disclaimer: The views expressed in this abstract are those of the authors and do not necessarily reflect the official policy of the Department of Defense or the U.S. Government.References
1. Muller J, Middleton D, Alizadeh M, et al. Hybrid diffusion imaging reveals altered white matter tract integrity and associations with symptoms and cognitive dysfunction in chronic traumatic brain injury. NeuroImage Clin. 2021;30:102681. doi:10.1016/j.nicl.2021.102681
2. Palacios EM, Owen JP, Yuh EL, et al. The evolution of white matter microstructural changes after mild traumatic brain injury: A longitudinal DTI and NODDI study. Sci Adv. 2020;6(32):eaaz6892. doi:10.1126/sciadv.aaz6892
3. Walker WC, Carne W, Franke LM, et al. The Chronic Effects of Neurotrauma Consortium (CENC) multi-centre observational study: Description of study and characteristics of early participants. Brain Inj. 2016;30(12):1469-1480. doi:10.1080/02699052.2016.1219061
4. Kay T, Harrington DE, Adams R, et al. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8(3):86-87. doi:10.1097/00001199-199309000-00010
5. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. Published online 2012. doi:10.1016/j.neuroimage.2012.03.072
6. Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009;62(3):717-730. doi:10.1002/mrm.22055
7. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53(6):1432-1440. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15906300
Figures