0466
Effect of iterative denoising in automated white matter hyperintensities segmentation using accelerated FLAIR sequences1Advanced Clinical Imaging Technology, Siemens Healthineers International AG, Lausanne, Switzerland, 2Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 3LTS5, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 4Department of Neuroradiology, Hospital and University of Montpellier, Montpellier, France, 5Institut d'Imagerie Fonctionnelle Humaine, I2FH, Hospital and University of Montpellier, Montpellier, France, 6Department of Neurology, Gui de Chauliac Montpellier University Hospital, Montpellier, Switzerland, 7Siemens Healthcare SAS, Saint-Denis, France
Synopsis
Keywords: Multiple Sclerosis, Segmentation, Fast MR Protocols
Acquisition time in brain MR protocols can be reduced using acceleration techniques like CAIPIRINHA. The inherent increase of noise due to their undersampling schemes can be mitigated with additional processing methods. Iterative denoising has shown good performance filtering images in k-space while preserving image details. This work evaluates the impact of iterative denoising on automated segmentation of white matter hyperintensities using CAIPIRINHA 3D FLAIR and compressed sensing 3D MPRAGE. Reliable segmentations were generated across different denoising levels (45% to 85%); small structures presented lower detection rates with stronger denoising (≥75%). These findings are revelant for designing optimized brain imaging protocols.Introduction
Recurrent challenges in medical image acquisition depend on three fundamental aspects: resolution, signal-to-noise ratio, and time. The MR scientific community has worked extensively on designing new sequences to reduce acquisition time while preserving quality even at high resolution1,2. These new techniques allow radiology departments to run accelerated protocols, leading to better patient experience and reduced costs. This is especially true for institutions facing an increasing number of patients where MRI is crucial for diagnosis and follow-up, such as in multiple sclerosis (MS)3,4. Brain MR acquisition guidelines for MS include 3D T1w and 3D FLAIR sequences3, which can make use of redundant coil information, like GRAPPA5 or CAIPIRINHA (CAIPI)6,7, or data sparsity as in compressed sensing8. These under sampling methods allow acceleration factors that often come at the expense of noise amplification or artifacts, thus requiring meticulous parametrization. Additional postprocessing can also complement the image reconstruction pipeline improving quality and preserving high acceleration factors. Iterative denoising is one of these techniques filtering the images with a configurable strength level directly in k-space, considering the spatial noise distribution in each scan9,10. Nevertheless, strong denoising can have an impact on the conspicuity of small structures, such as white matter hyperintensities (WMH), especially when computing quantitative measurements provided by automated methods trained on data acquired with conventional sequences. This work explores the effect of the denoising level in optimized CAIPI FLAIR sequences10 and its impact on the automated WMH detection using a research application developed in-house11,12. We compare the results to the ones obtained with a GRAPPA-accelerated FLAIR widely used in conventional protocols.Materials and Methods
Whole-brain 3D T1w and 3D FLAIR images were collected from 18 MS patients (12 female, median age = 40 years, range = [19, 59] years) scanned at 3T (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). Each study included a compressed sensing MP-RAGE sequence, a GRAPPA 3D FLAIR and five reconstructed CAIPI 3D FLAIR series with increasing levels of denoising (45% to 85%, details of the protocols in Table 1).The WMH segmentation tool relies on 3D MPRAGE and 3D FLAIR sequences to generate a mask of WMHs. It provides WMH volumes in mL and count for the whole brain, and their estimation in four regions: periventricular, juxtacortical, infratentorial and deep white matter. A reference segmentation was used to compute the WMH detection accuracy. For each patient, a precomputed binary mask was edited by two reviewers (a research engineer and a neuroradiologist consecutively, both with more than 5 years of experience in MS) considering the GRAPPA FLAIR and the T1 MPRAGE sequence as reference. F1-scores were computed to assess detection accuracy. Paired sample t-tests of WMH volume and count were evaluated between all possible combinations of results obtained with the six FLAIR sequences. Shapiro-Wilk tests were run to verify normality of the differences, p-values were adjusted for false discovery rate in multiple comparisons using Benjamini-Hochberg approach.Results
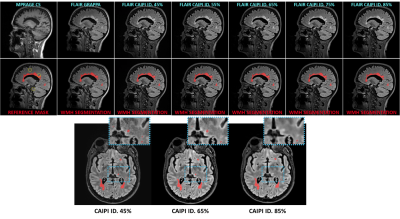
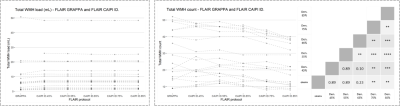
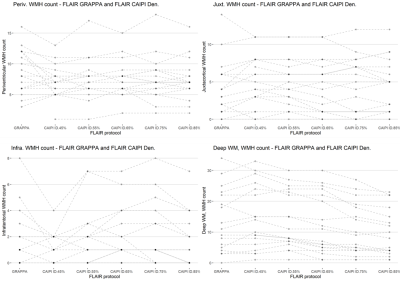
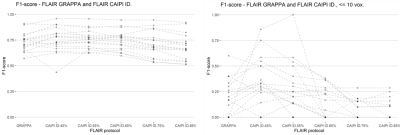
Figure 1 shows slices of all sequences available for each patient, the corresponding automated and reference WMH segmentation masks. Patients showed a large variability in total WMH load (median [min., max.]: 5.73 [0.91, 50.76] mL) and count (median [min., max.]: 24 [9, 52]), but there were no significant differences on WMH volumes derived from GRAPPA FLAIR and any of the denoised CAIPI FLAIR sequences. Conversely, there were significant differences in WMH count with increasing levels of denoising, particularly above 75% compared to GRAPPA FLAIR or lower denoising levels (Figure 2). Regional differences in WMH count were mainly found in the deep WM, although other regions have a lower number of WMHs (Figure 3). Iterative denoising did not have a significant impact on overall WMH detection. The median F1-score using GRAPPA FLAIR was 0.73; for CAIPI FLAIR with denoising levels from 45% to 85%: 0.76, 0.76, 0.76, 0.69, 0.67. No significant differences in detection were found, but there was a slight decrease in detection with higher denoising levels particularly >75%. It was confirmed that WMH of less than 10 voxels were detected less often with higher denoising, but also the lowest denoising presented a higher detection (0.32) than the GRAPPA FLAIR (0.17) despite the increased noise visually perceived in the image (Figure 4).Discussion and Conclusions
Iterative denoising can considerably help to improve image quality, especially at higher resolutions and higher acceleration factors. The right parametrization of the denoising is crucial for a reasonable compromise between good image quality for visual clinical assessment and performance of automatic detection algorithms. This study shows that an example for automated WMH segmentation is sensitive to strong denoising levels (75% and higher); additionally, lower levels may be beneficial for detecting small structures without a substantial increase in false positives. The algorithm shows robustness on global estimation of WMH load; more analyses are needed to confirm the generalizability of our observations with other WMH segmentation algorithms and to assess the variability of WMH count in longitudinal assessments, a context where varying MR parameters across time-points are very likely to occur. Finally, we suggest that introduction of new sequences and/or acceleration techniques should trigger explicit validations of automated algorithms, especially with data-driven algorithms that are common nowadays.Acknowledgements
We thank patients for their participation in this study, as well as the medical staff supporting the MR image acquisition at the University Hospital in Montpellier.References
1. Hamilton J, Franson D, Seiberlich N. Recent advances in parallel imaging for MRI. Prog Nucl Magn Reson Spectrosc. 2017; 101:71-95.
2. Sheng J, Shi Y, Zhang Q. Improved parallel magnetic resonance imaging reconstruction with multiple variable density sampling. Sci Rep. 2021 Apr 26;11(1):9005
3. Wattjes M P, Cicarelli O, Reich D S, et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021; 20(8):653‐670.
4. Brisset J C, Kremer S, Hannoun S, et al. New OFSEP recommendations for MRI assessment of multiple sclerosis patients: Special consideration for gadolinium deposition and frequent acquisitions. J Neuroradiol. 2020; 47(4):250-8.
5. Griswold M A, Jakob P M, Heidemann R M, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn. Reson. Med. 2002; 47:1202–1210.
6. Breuer FA, Blaimer M, Mueller MF, et al. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magn Reson Med. 2006; 55:549–556.
7. Breuer FA, Moriguchi H, Seiberlich N, et al. Zigzag sampling for improved parallel imaging. Magn Reson Med. 2008 Aug; 60(2):474-8.
8. Forman C, Wetzl J, Hayes C, Schmidt M. Compressed Sensing: a Paradigm Shift in MRI. MAGNETOM Flash 2016; 66: 8-13
9. Eliezer M, Vaussy A, Toupin S, et al. Iterative denoising accelerated 3D SPACE FLAIR sequence for brain MR imaging at 3T. Diagn Interv Imaging. 2022; 103(1):13-20
10. Vaussy A, Eliezer M, Menjot de Champfleur N, et al. Iterative denoising applied to 3D SPACE CAIPIRINHA: a new approach to accelerated 3D brain examination in clinical routine. MAGNETOM Flash, Free.Max special issue 2020; 26-34
11. Fartaria MJ, Bonnier G, Roche A, et al. Automated detection of white matter and cortical lesions in early stages of multiple sclerosis: Automated MS Lesion Segmentation. J Magn Reson Imaging. 2016; 43(6):1445-54.
12. Fartaria MJ, Todea A, Kober T, et al. Partial volume-aware assessment of multiple sclerosis lesions. NeuroImage Clin. 2018. 18:245-253
Figures