0455
Exploring whether differences in brain diffusion MRI metrics distinguish symptom phenotypes in athletes exposed to repetitive head impacts.1Mātai Medical Research Institute, Tairāwhiti-Gisborne, New Zealand, 2Department of Anatomy and Medical Imaging, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand, 3Auckland Bioengineering Institute, University of Auckland, Auckland, New Zealand, 4Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand, 5Faculty of Medical and Health Sciences & Centre for Brain Research, University of Auckland, Auckland, New Zealand, 6Ngāti Porou, Ngāti Kahungunu, Rongomaiwahine, Rongowhakaata, Tairāwhiti-Gisborne, New Zealand, 7Ngai Tāmanuhiri, Rongowhakaata, Ngāti Porou, Tairāwhiti, New Zealand, 8Turanga Health, Tairāwhiti-Gisborne, New Zealand, 9School of Computer Science, Faculty of Science, University of Auckland, Auckland, New Zealand, 10General Electric Healthcare, Victoria, Australia, 11Department of Psychology and Neuroscience, Auckland University of Technology, Auckland, New Zealand
Synopsis
Keywords: Traumatic brain injury, Diffusion Tensor Imaging, Symptomology
It is important to account for the heterogeneity of clinical presentation when studying mild traumatic brain injury (mTBI) with advanced brain imaging. We used post-season symptom data from a cohort of rugby players exposed to repetitive head impacts, and we applied unsupervised learning to cluster athletes into clinically distinct groups. We explored whether these clusters demonstrated post-season group differences in white matter tracts compared to controls. This analysis framework suggests that group differences of diffusion metrics in athletes exposed to repetitive head impacts may be associated with clinical presentation rather than generalisable across all participants.Introduction
Mild traumatic brain injury (mTBI) represents a spectrum of biomechanically induced brain injury, spanning single exposure events that result in a clinically diagnosed injury to the accumulation of mild, repetitive head impacts where symptomology is present despite no single impact resulting in a diagnosis1,2. There are no valid objective biomarkers for mTBI. Consequently, management is guided by subjective symptom reports and clinical examination3. Advanced neuroimaging, such as diffusion MRI, has demonstrated sensitivity to detect changes in white matter tracts following repetitive exposure to mTBI4. The clinical presentation of mTBI is highly heterogenous5. Yet, there is a lack of imaging studies incorporating clinical measures of mTBI to interpret the practical meaningfulness of differences detected by imaging. Adopting statistical frameworks that use clinical measures to account for the heterogeneity of mTBI presentation when analysing imaging data is critical to advancing our knowledge of mTBI. Here, we use symptom and diffusion MRI data from a cohort of rugby players exposed to repetitive head impacts throughout a season to demonstrate a framework using hierarchical clustering of mTBI symptom data to guide group analysis of diffusion MRI. We hypothesised that clustering would reveal clinically distinct clusters of athletes with high and low symptomology; compared to controls, differences in white matter tracts would only be observed in the high symptom burden cluster.Methods
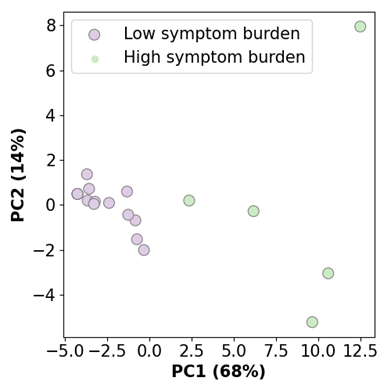
Thirty-seven male high school rugby players (15 - 18 years old) were recruited for this study and monitored over the course of the competitive season. Analyses were performed on post-season data from a subset of 20 rugby players and 11 representative controls who do not play collision sports. Athletes and controls completed the Brain Injury Screening Tool (BIST) to quantify symptoms commonly associated with mTBI6. Images were acquired using a 3T MRI scanner (SIGNA Premier; General Electric Healthcare, Milwaukee, WI) with an AIR™ 48-channel head coil. Multi-shell diffusion MRI scans (b-values = 0, 1000, 2000, 3000 s/m2; 54 gradient directions=4,15,15,20 respectively; 4 b=0; 2mm isotropic voxel size) were acquired on the brains of all participants to investigate the structural changes in white matter integrity. MRtrix7 and TractSeg8 were used to segment three bundles of fibre tracts (Figure 1) and measure the diffusion indices (FA, MD, AD, RD). Hierarchical clustering was performed on all symptom data to identify two clusters of athletes with high versus low symptom burden following a season of repetitive head impact exposure. Symptom cluster membership was visualised by applying Principal Component Analysis to the symptom data and plotting PC1 and PC2 with cluster labels. Differences in symptomology between the clusters and the control group were evaluated using Mann-Whitney U tests. Finally, Mann-Whitney U tests were performed on diffusion indices between the two clusters of rugby players and the control group to explore whether white matter differences underly mTBI symptomology. The False Discovery Rate (FDR) method accounted for multiple comparisons across the imaging results.Results
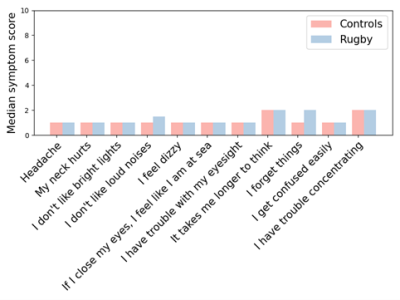
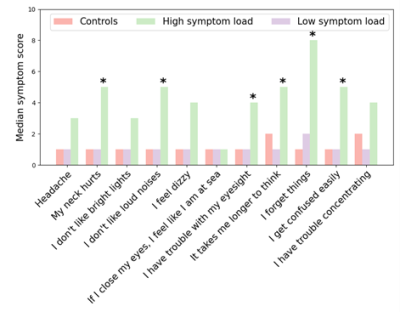
Before clustering, no differences were observed between rugby players and controls for any 11 BIST symptoms (Figure 2). After clustering, five and 15 rugby players were grouped into high and low symptom burden clusters, respectively (Figure 3). No differences in symptoms were apparent between the low symptom burden cluster and controls. Significantly higher symptomology was detected between the high symptom burden cluster and controls suggesting these clusters represent clinically distinct phenotypes (Figure 4). Counter to our hypothesis, no differences in FA, MD, AD, RD were observed between the high symptom burden cluster and the controls within the tracts of interest. Instead, compared to controls, the low symptom burden cluster exhibited: significantly decreased AD for the corpus callosum (p = 0.019, uncorrected), right (p = 0.009, uncorrected) and left (p = 0.022, uncorrected) corticospinal tracts; decreased MD of the right (p = 0.009, uncorrected) and left (p = 0.010, uncorrected) cingulum; and decreased RD in the right cingulum (p = 0.031, uncorrected). None of these differences remained significant after accounting for multiple comparisons.Discussion/Conclusion
We have presented a framework demonstrating the importance of accounting for clinically distinct phenotypes while analysing neuroimaging data collected from individuals exposed to mTBI. While the observed group differences were counter to our hypothesis, these findings reveal that – compared to controls – differences in white matter tracts after repetitive head impacts may be specific to mTBI symptom burden.This work is preliminary, illustrating how clinical data can be leveraged in neuroimaging investigations of mTBI. This framework can be applied to other imaging modalities, such as functional or perfusion MRI. Given that symptom data can be acquired quickly and at little to no cost, future mTBI neuroimaging studies should strongly consider measuring symptomology at the time of scanning. If neuroimaging is to advance our knowledge of the consequences of mTBI, we need to integrate the clinical and symptomatological importance of imaging findings statistically.
Acknowledgements
This work was supported by Kānoa - Regional Economic Development & Investment Unit, New Zealand; the Catalyst Strategic Fund from Government Funding administered by the New Zealand Ministry of Business Innovation and Employment; and the Hugh Green Foundation. We are grateful to Mātai Ngā Māngai Māori for their guidance and to our research participants for dedicating their time toward this study. We would like to acknowledge the support of GE Healthcare.References
1. Carroll, L.J., et al., Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med, 2004(43 Suppl): p. 113-25.
2. Mayer, A.R., D.K. Quinn, and C.L. Master, The spectrum of mild traumatic brain injury: A review. Neurology, 2017. 89(6): p. 623-632.
3. Silverberg, N.D., et al., Management of Concussion and Mild Traumatic Brain Injury: A Synthesis of Practice Guidelines. Arch Phys Med Rehabil, 2020. 101(2): p. 382-393.
4. Tayebi, M., et al., The role of diffusion tensor imaging in characterizing injury patterns on athletes with concussion and subconcussive injury: a systematic review. Brain Inj, 2021. 35(6): p. 621-644.
5. Iverson, G.L., et al., Predictors of clinical recovery from concussion: a systematic review. British Journal Of Sports Medicine, 2017. 51(12): p. 941-948.
6. Theadom, A., et al., The Brain Injury Screening Tool (BIST): Tool development, factor structure and validity. PLOS ONE, 2021. 16(2): p. e0246512.
7. Tournier, J.D., et al., MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 2019. 202: p. 116137.
8. Wasserthal, J., P. Neher, and K.H. Maier-Hein, TractSeg - Fast and accurate white matter tract segmentation. Neuroimage, 2018. 183: p. 239-253.
Figures