0453
Repetitive Mild Traumatic Brain Injury Induces Significant Changes on Functional Connectivity with Different Impact Numbers in Rats1Department of Biomedical Imaging and Radiological Sciences, National Yang-Ming Chiao-Tung University, Taipei, Taiwan, 2Department of Medical Imaging and Radiological Sciences, Chang Gung University, Taoyuan, Taiwan, 3Department of Medical Imaging and Intervention, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan
Synopsis
Keywords: Traumatic brain injury, Animals, rsfMRI
In the current study, we discussed about changes of brain connectivity through rsfMRI and behavioral performances following repetitive mild traumatic brain injury (rmTBI) with different impact numbers and intervals. In rats with larger impact number but longer inter-injury interval, may show anxiety-like behavior with the significant reduction of connectivity in their DMN.
Introduction
Mild traumatic brain injury (mTBI) is an important global health issue and often occurs in military or sports field and the repetitive mTBI (rmTBI) accounts for a considerable portion leading to cumulative damage to the brain.1 Resting-state fMRI (rsfMRI) documenting brain changes has been emerged to be a useful tool measuring functional connectivity post-TBI.2 The default mode network (DMN), an important functional network associated with many psychiatric brain disorders and anxiety-like performances, has found to be linked to post-mTBI symptoms such as depression or agoraphobia.3,4,5 Despite the privilege of studying brain disorders on rsfMRI, to date, there’s limited research done on the related field, due to the difficulties of building reliable animal models under rsfMRI.6 Besides, studies in rmTBI animal models are also limited compared with the single mTBI (smTBI).7 To this aim, we mainly focused on exploring the effect of multiple impacts on the brain connectivity using the model of rmTBI. Moreover, we also evaluated the locomotor activity, as well as the anxiety-like behavior, in rats after mTBI.Methods
A total of 30 male SD rats were used in this study and randomly divided into 4 experimental groups: (1) sham (n=15) (2) single mTBI (smTBI, n=5) (3) rmTBI of 2 impacts with 1 h interval (n=5) (4) rmTBI of 3 impacts with 6 h intervals (n=5). Rats were anesthetized with isoflurane (0.2-0.25 %) for sham surgery, and mTBI groups further underwent stereotaxic surgery. To mimic the uncomplicated mTBI, the closed-head injury (CHI) model was performed.8 In brief, a weight of 600 g was dropped from a height of 1 m through a stainless-steel tube to the secured impactor aiming to the metal helmet cemented on the central of the skull (3.5 mm posterior to bregma). All rats underwent MRI scanning at a single time point (sham at day 0 and mTBI groups at day 50 after mTBI) using a Bruker 7T Pharma Scan. After the bolus injection of dexmedetomidine (0.015 mg/kg), continuous infusion of dexmedetomidine (0.03 mg/kg/h) was performed with low-dose isoflurane (0.25%) during the whole MR protocol.9 rsfMRI was performed after 90 min over infusion and acquired using the single-shot echo-planar imaging (EPI; TR/TE=3000/17.5 ms; matrix size= 96 × 96; FOV=3.5 x 3.5 cm; slice thickness=1 mm) as described in the previous study.10 T2-weighted images were also acquired with the same geometry (TR/TE=3000/30 ms; matrix size= 256 × 256). rsfMRI data was analyzed and represented with group ICA maps in this study.11 The Waxholm Space (WHS) Rat Brain atlas and the Paxinos and Watson rat brain atlas were used to define the region of interests (ROIs) from the extracted ICs. Open-field test for 10 min was designed to examine behavioral changes in subgroup of animals (n=5 for each group) after MRI acquisition. Quantitative behavior outcomes including the total distance traveled and the time in zones were tracked. One-way ANOVA and Kruskal-Wallis test were used to determine the differences in connectivity within the network, as well as the behavioral parameters, among the groups (P < 0.05).Results and Discussions
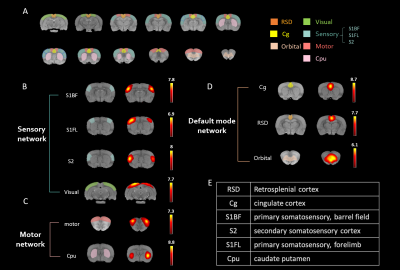
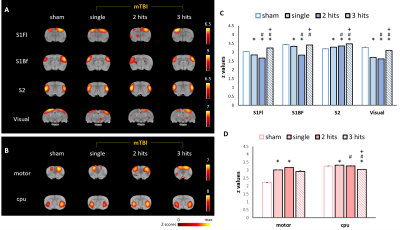
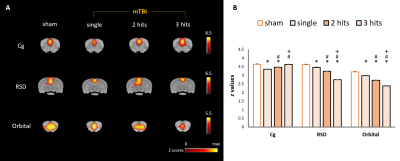
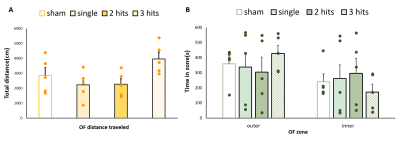
In this study, the major ICs of 9 region of interest (ROIs) including (1) the sensory network with the barrel field region (S1BF), the forelimb region (S1FL), the secondary somatosensory cortex (S2) and the visual cortex (Visual), (2) the motor network with the motor cortex (Motor) and the caudate putamen (CPu), and (3) the default mode network (DMN) in rats with the cingulate cortex (cg), the retrosplenial cortex (RSD), and the orbitofrontal cortex (Orbital) were identified (Fig. 1). In sensory network, significant lower connectivity strength within the S1Fl, S2, and Visual were observed as the impact number increases from single to two CHIs within 1 h. However, the connectivity further recovered when the impact interval elongated to 6 h with 3 CHI hits (P<0.05, Fig. 2C). Significant increment of connectivity strength was observed in the single and two CHIs groups in the motor cortex (P<0.05, Fig. 2D) but not in the animals with 3 CHI hits. In DMN, significant decrease in connectivity was observed within RSD and orbital along with the number of impacts, while the connectivity within Cg increased (P<0.05, Fig. 3B). The rats were also tested for alterations in behavior through open field test, a measure of overt locomotor activity and anxiety like behaviors12,13, at the 50 days after brain injury. Although the animals after mTBI showed no significant motor deficits, there was a trend that the animals receiving 3 hits might travel further compared with other smTBI or rmTBI animals (Fig. 4B). In addition, no significant changes in anxiety-like behavior was observed after mTBI (Fig. 4B). Nevertheless, the rats with 3 CHI hits may still be more anxious as they stayed more time in the outer zone, which may also correlate to the reduction in connectivity within RSD in DMN.14,15 Our future studies will aim to explore the changes in between-network connectivity and to correlate functional connectivity in the brain with the behavioral outcomes after mTBI.Acknowledgements
This study was funded in part by Ministry of Science and Technology (NSTC 111-2314-B-A49-086 and MOST 109-2314-B-010-067-MY3), Taipei,Taiwan.References
1. Weber JT. Experimental models of repetitive brain injuries. Prog Brain Res. 2007;161:253-61.
2. Huang S, Shen Q, Watts LT, Long JA, O'Boyle M, Nguyen T, et al. Resting-State Functional Magnetic Resonance Imaging of Interhemispheric Functional Connectivity in Experimental Traumatic Brain Injury. Neurotrauma Rep. 2021;2(1):526-40.
3. Sharp DJ, Beckmann CF, Greenwood R, Kinnunen KM, Bonnelle V, De Boissezon X, et al. Default mode network functional and structural connectivity after traumatic brain injury. Brain. 2011;134(Pt 8):2233-47.
4. Walker WC, Franke LM, McDonald SD, Sima AP, Keyser-Marcus L. Prevalence of mental health conditions after military blast exposure, their co-occurrence, and their relation to mild traumatic brain injury. Brain Inj. 2015;29(13-14):1581-8.
5. Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463(1-3):3-33.
6. Pan WJ, Billings JC, Grooms JK, Shakil S, Keilholz SD. Considerations for resting state functional MRI and functional connectivity studies in rodents. Front Neurosci. 2015;9:269.
7. Hoogenboom WS, Branch CA, Lipton ML. Animal models of closed-skull, repetitive mild traumatic brain injury. Pharmacol Ther. 2019;198:109-22.
8. Kao YJ, Lui YW, Lu CF, Chen HL, Hsieh BY, Chen CY. Behavioral and Structural Effects of Single and Repeat Closed-Head Injury. AJNR Am J Neuroradiol. 2019;40(4):601-8.
9. Lu H, Zou Q, Gu H, Raichle ME, Stein EA, Yang Y. Rat brains also have a default mode network. Proc Natl Acad Sci U S A. 2012;109(10):3979-84.
10. Kao Y C J L, C.F.; Chen, C.Y., Behavioral and Image Evidence for Mild Traumatic Brain Injury in Rats with the Skull Helmet ISMRM Preceeding, 2017.
11. Kao YJ, Lu CF, Tsai PH, Hsu FT, Hsieh BY, Chen CY. Low-frequency Fluctuations of Resting-state fMRI BOLD Signal after Experimental Mild Traumatic Brain Injury. ISMRM Preceeding, 2018.
12. Walsh RN, Cummins RA. The Open-Field Test: a critical review. Psychol Bull. 1976;83(3):482-504.
13. Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463(1-3):3-33.
14. Coutinho JF, Fernandesl SV, Soares JM, Maia L, Gonçalves Ó F, Sampaio A. Default mode network dissociation in depressive and anxiety states. Brain Imaging Behav. 2016;10(1):147-57.
15. Oyarzabal EA, Hsu LM, Das M, Chao TH, Zhou J, Song S, et al. Chemogenetic stimulation of tonic locus coeruleus activity strengthens the default mode network. Sci Adv. 2022;8(17):eabm9898.
Figures