0436
Endometriosis targeted MRI imaging using bevacizumab-modified nanoparticles aiming at vascular endothelial growth factor1Department of Radiology, Huashan hospital, Fudan University, Shanghai, China, 2MR Application Development, Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China, 3MR Collaborations, Siemens Healthineers Digital Technology (Shanghai) Co., Ltd., Shanghai, China, 4Fudan University, Shanghai, China
Synopsis
Keywords: Uterus, Molecular Imaging
In vivo, NPBCNs generated strong signal enhancement in endometriosis lesion in rat on T1-weighted images via MRI.Abstract
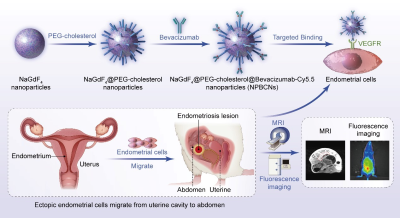
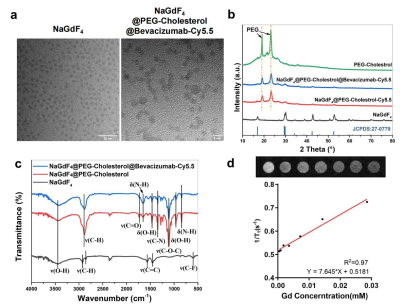
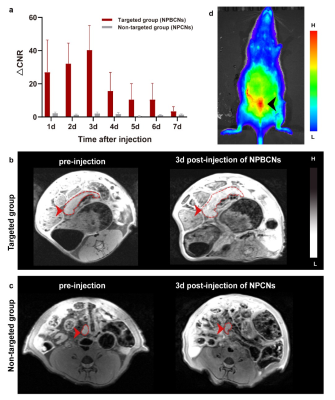
Introduction: More than 175 million women worldwide have been suffering from endometriosis and its complications [1]. The clinical gadolinium (Gd)-based MRI contrast agents is used for enhancing the visibility of interested tissue[2]. However, as endometriosis exhibit infiltrating growth without evident boundary, clinical MRI contrast agents are non-targeting ,and thus cannot reveal the whole scope for precise surgical plan making. Therefore, targeted MRI contrast agent for endometriosis would effectively help clinicians delineate the extent of surgical resection precisely. Materials and Methods: The synthesizing process of two kinds of nanoparticles, NPBCNs and NPCNs was illustrated in Figure 1. First, oleic acid-stabilized NaGdF4 nanoparticles were synthesized through a thermal decomposition process as previously described[19], and subsequently modified with PEG-cholestrol, bevacizumab and Cy5.5 for hydrophilicity, endometriosis‐targeting and fluorescence tracking respectively. Transmission electron microscopy (TEM) images (Figure 2a) confirmed the successful preparation of NaGdF4 nanoparticles and NPBCNs, demonstrating uniform spherical morphology and excellent dispersibility. The crystallite size and phase formation of NaGdF4 nanoparticles, NaGdF4 nanoparticles, NaGdF4@PEG-cholesterol-Cy5.5 nanoparticles (NPCNs) and NPBCNs were identified and confirmed by comparing the data with standard X‐ray diffraction (XRD) patterns from JCPDS (Figure 2b). The diffraction peaks located at 29.8°, 42.5° and 52.5° of the NPBCNs and NPCNs corresponded with the characteristic (101), (201) and (211) diffraction peaks of the NaGdF4 standard spectrum, respectively. The broad XRD peaks of NPBCNs and NPCNs around 18 and 23° were associated with PEG crystal. Furthermore, Fourier transform infrared (FT‐IR) spectra verified the successful surface modification (Figure 2c), especially the stretching vibration of the C=O at 1734 cm-1, vibrations of the C-O-C at 1113 cm-1 as well as out-of-plane bending vibration of O-H around 963 cm-1. The T1 imaging ability of NPBCNs, the relaxivity (r1) of NPBCNs was measured on 3-T MR platform and the analytic results was plotted in the Figure 2d. Results: As Figure 3a shown, the dynamic change of the two groups after injection of NPBCNs or NPCNs was investigated quantitatively. The CNR of endometriosis lesion was calculated as the difference in signal-to-noise ratio between endometriosis lesion and muscle regions of interest. The time dependence of the change in CNR (ΔCNR=CNRpre - CNRpost) following injection was determined from the sequential MR images. The ΔCNR of the endometriosis lesion in the targeted group was higher than non-targeted group on 1d post-injection and gradually increased, then reached to a peak at 3d post‐injection. The difference between the targeted group (40.26±14.94) and non-targeted group (2.12± 0.84) was approximately maximal 3 days after NPBCNs injection. Besides, ΔCNR value of the targeted group at each time point was statistically significantly higher than that of the non-targeted group from 1d to 7d post‐injection (P=0.02 < 0.05). The enhancement in the targeted group then began to weaken but sustained visibly until 6d post‐injection. The time-dependent ΔCNR data was used to determine the optimal imaging time point where the maximal ΔCNR is observed. Herein, the 3d-post injection could set as the optimal imaging time point for the image comparation between the two groups. T1 signal of the endometriosis lesions in the targeted group manifested obvious enhancement 3 days after NPBCNs injection (Figure 3b). In contrast, in the non-targeted group, no contrast‐enhanced region inside endometriosis tissue was observed (Figure 3c), or in other tissue following NPCNs injection. It is known that the enhanced permeability and retention (EPR) effect would result in deposition of non-targeted nanoparticles in tumor[3,4]. Notably, as the endometriosis is non-tumor disease, the non-targeted NPCNs could not accumulate in endometriosis to generate visible enhancement on T1-weighted images. In summary, the T1 signal enhancement in the targeted group was resulted from the targeted ability of NPBCNs towards VEGF signaling pathway of endometriosis. Discussion: NPBCNs synthesized in our study served as a novel MRI contrast agent, which exhibited high relaxation rate and excellent specificity for endometriosis, filling the blank of targeted imaging diagnosis for endomtriosis. Owing to its high affinity to VEGF receptor in ectopic endometrial cells, NPBCNs can serve as an efficient MR imaging probe for the accurate detection and targeted imaging of endometriosis lesions. Moreover, it can also be shown on fluorescent images in vivo. More than that, as neovascularity is highly associated with proliferation of endometriosis, the T1 signal intensity caused by NPBCNs may be used to quantify the proliferative activity of multiple endometriosis foci and classify the priority during surgical plan in future studies. Additionally, no obvious toxicity and adverse effect of NPBCNs was observed, demonstrating their great biocompatibility. Therefore, it can be potentially used in the clinical endometriosis lesion detection by MRI. It is postulated that NPBCNs have potential application in accurately medical diagnosis of endometriosis.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81671732).References
1. David Adamson G, Kennedy S, Hummelshoj L. Creating solutions in endometriosis: Global collaboration through the World Endometriosis Research Foundation. J Endometr. 2010;2:3–6.
2. Liu Z, Zhao M, Wang H, Fu Z, Gao H, Peng W, et al. High relaxivity Gd3+-based organic nanoparticles for efficient magnetic resonance angiography. J Nanobiotechnology. 2022;20.
3. Ni D, Jiang D, Im HJ, Valdovinos HF, Yu B, Goel S, et al. Radiolabeled polyoxometalate clusters: Kidney dysfunction evaluation and tumor diagnosis by positron emission tomography imaging. Biomaterials. 2018;171:144–52.
4. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release. 2000;65:271–84.
Figures