0432
Characterization of age- and gender-dependent differences in the inter-vertebral disc using MR-Fingerprinting and Textural Analysis1Radiology, NYU Grossman School of Medicine, New York, NY, United States
Synopsis
Keywords: MR Fingerprinting/Synthetic MR, Tissue Characterization, degenerative
The study investigated age and gender differences in intervertebral disc (IVD) of the lumbar spine using a multiparameter MR fingerprinting (MRF) technique that simultaneously quantify T1, T2 and T1ρ. Seventeen healthy subjects were evaluated. There was a significant positive association between age and T1ρ of the IVD and between age and various gray level co-occurrence matrix features of T1 maps on texture analysis. There were no significant differences between males and females in T1, T2 and T1ρ of the IVD. The results demonstrate that multi-parameter MRF can effectively characterize early age-related IVD degeneration.
INTRODUCTION
Discogenic back pain is a common and debilitating musculoskeletal condition, which is primarily due to degenerative changes in the inter-vertebral disc (IVD)1,2. Quantitative MR parameters such as T1, T2 and T1ρ are used together in research studies for comprehensive assessment of IVD composition and structure but require independent acquisitions, which results in long scan times. MR fingerprinting (MRF) has allowed the development of simultaneous multi-parameter mapping that could be used for more time-efficient, comprehensive characterization of the IVD. This study was performed to investigate age and gender differences in IVD using a multi-parameter MRF technique that can simultaneously quantify T1, T2 and T1ρ in a clinically feasible scan time.METHODS
The study cohort consisted of 17 healthy subjects (9 female (34±10yrs), 8 male (34±10yrs), age range=20-60yrs) who underwent an MRF scan of the lumbar spine using a 3T-MRI scanner (Prisma, Siemens Healthcare, Germany).A 3D implementation of the MRF sequence is shown in Figure 13,4. An adiabatic inversion pulse is followed by two FISP segments that encode for T1/T2, where each FISP segment consists of 250 RF excitations and two FLASH segments that encode for T1/B1. The flip angles (FA) for the FISP and FLASH segments vary from 0° to 20°, and from 0° to 60° for the first and second segments, respectively. This is followed by a T1ρ preparation module followed by 125 RF excitations for each spin lock pulse, with FA’s ranging from 0° to 20°. This implementation used 6 spin lock pulses at TSL=2, 4, 7, 13, 25, 45 ms. Golden angle radial readouts following each RF excitation was used with centric out readout in the kz dimension.
The 3D-MRF sequence acquired 10 sagittal slices of the lumbar spine with 4 shots in 10mins. The common MR acquisition parameters included: FOV=240mm, orientation= sagittal, in-plane voxel resolution= 0.7x0.7 mm2, 4 mm through plane slice thickness, TR=7.5ms, TE=3.5 ms, bandwidth=500Hz/pixel, frequency of spin lock = 500 Hz. Extended phase graph5 simulations were performed to compute a dictionary of simulated MR fingerprints with a T1 range of 50-3000ms, T2 range of 2-200ms, and T1ρ range of 2-200ms in steps of 6%. SVD compression was used to speed up the reconstruction5, which was performed offline. An iterative dictionary pattern matching algorithm was used to produce quantitative maps of proton density (PD), T1, T2 and T1ρ and B1.
Manual segmentation of the IVD segments was performed. Mean global T1, T2 and T1ρ and mean T1, T2 and T1ρ of individual IVDs were measured. Gray level co-occurrence matrix (GLCM) features6 were used to quantify global textural features of the IVD from the T1, T2 and T1ρ maps.
Kruskal-Wallis and Wilcoxon rank tests were used to assess the association between T1, T2 and T1ρ of the IVD and age (4 groups: 20-30yrs, 31-40yrs, 41-50yrs, 51-60yrs) and gender (males vs. females), respectively. Spearman correlation tests were used to assess the association between age and mean T1, T2 and T1ρ and global textural features of T1, T2 and T1ρ maps of the IVD. Statistical significance was set to 0.05.
RESULTS
Figure 2 shows representative images from multi-parameter maps for PD, T1, T2 and T1ρ for each age group and gender. As shown in Figure 3, there were significant positive correlations between age and global T1, T2 and T1ρ of the IVD. However, T1ρ had the strongest positive association with age with the highest correlation coefficients, significant correlations for the individual IVDs, and significant differences between the 4 age groups. As shown in Figure 4, there was no significant differences between males and females in global T1, T2 and T1ρ of the IVD. Figure 5 shows the results of the textural analysis. There was a significant inverse correlation between age and global mean T1, T2 and T1ρ texture of the IVD and significant correlations between age and global GLCM features on the T1 maps.DISCUSSION
Our study showed significant age differences but non-significant gender differences in T1, T2 and T1ρ of the IVD of the lumbar spine. Asymptomatic IVD degeneration typically begins in the third decade of life and is characterized by decreased proteoglycan content and increased water content within the nucleus pulposus7-9. T1ρ of the IVD had the strongest positive correlation with age in our study, which suggests that decreased proteoglycan content of the IVD measured with T1ρ is more strongly affected during the aging process than increased free water content measured with T2. Global GLCM features on T1 maps also showed significant moderate correlations with age. This likely reflects changes in the macromolecular structure of the IVD, which occurs especially in the annulus fibrosis during the early stages of IVD degeneration and eventually lead to IVD clefts and tears7-9. The absence of significant gender-related differences in T1, T2 and T1ρ in our study suggests that baseline differences in IVD composition and structures are not responsible for potential gender-difference differences in discogenic back pain.CONCLUSION
Our study demonstrates that multi-parameter MRF can effectively characterize early IVD degeneration, with T1ρ being especially useful for detecting age-related IVD changes.Acknowledgements
This study was supported by NIH grants, R21-AR078357, R01-AR076328-01A1, R01-AR076985-01A1, and R01-AR078308-01A1 and was performed under the rubric of the Center of Advanced Imaging Innovation and Research (CAI2R), an NIBIB Biomedical Technology Resource Center (NIH P41-EB017183).References
1 Fujii, K. et al. Discogenic Back Pain: Literature Review of Definition, Diagnosis, and Treatment. JBMR Plus 3, e10180, doi:10.1002/jbm4.10180 (2019).
2 Bouhsina, N. et al. Comparison of MRI T1, T2, and T2* mapping with histology for assessment of intervertebral disc degeneration in an ovine model. Sci Rep 12, 5398, doi:10.1038/s41598-022-09348-w (2022).
3 Sharafi, A., Zibetti, M. V. W., Chang, G., Cloos, M. & Regatte, R. R. MR fingerprinting for rapid simultaneous T1 , T2 , and T1 rho relaxation mapping of the human articular cartilage at 3T. Magn Reson Med 84, 2636-2644, doi:10.1002/mrm.28308 (2020).
4 Sharafi, A. et al. Simultaneous T1 , T2 , and T1rho relaxation mapping of the lower leg muscle with MR fingerprinting. Magn Reson Med 86, 372-381, doi:10.1002/mrm.28704 (2021).
5 McGivney, D. F. et al. SVD Compression for Magnetic Resonance Fingerprinting in the Time Domain. Ieee Transactions on Medical Imaging 33, 2311-2322, doi:10.1109/Tmi.2014.2337321 (2014).
6 Menezes-Reis, R. et al. Lumbar intervertebral discs T2 relaxometry and T1rho relaxometry correlation with age in asymptomatic young adults. Quant Imaging Med Surg 6, 402-412, doi:10.21037/qims.2016.08.01 (2016).
7 Pearce, R. H., Grimmer, B. J. & Adams, M. E. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res 5, 198-205, doi:10.1002/jor.1100050206 (1987).
8 Antoniou, J. et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest 98, 996-1003, doi:10.1172/JCI118884 (1996).
9 Singh, K., Masuda, K., Thonar, E. J., An, H. S. & Cs-Szabo, G. Age-related changes in the extracellular matrix of nucleus pulposus and anulus fibrosus of human intervertebral disc. Spine (Phila Pa 1976) 34, 10-16, doi:10.1097/BRS.0b013e31818e5ddd (2009).
Figures
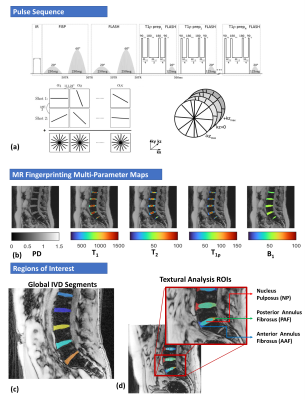
Figure 1: The top image (a) shows the 3D-MR fingerprinting (MRF) pulse sequence that was used to simultaneously quantify T1, T2 and T1ρ of the IVD. (b) shows representative results obtained after iterative reconstruction and pattern matching. (c) shows regions of interest of the individual IVDs that were manually segmented.
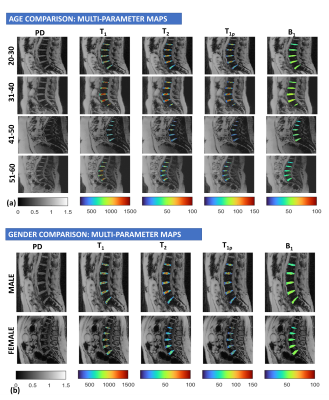
Figure 2: Representative images from multi-parameter maps for PD, T1, T2 and T1ρ for each age group (a) and (b) gender.

Figure 3: (a) Results showing variations of T1, T2 and T1ρ parameter maps with age. (b) The Kruskal Wallis tests show significant differences between the 4 different age group for T1ρ of the IVDs. (c) The regression plots and (d) Spearman correlations show significant correlation between age and global T1, T2 and T1ρ of the IVD and between age and T1ρ for the individual IVDs.
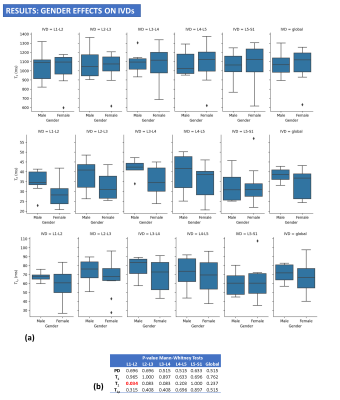
Figure 4: (a) Results showing variations of T1, T2 and T1ρ parameter maps with gender. (b) Mann-Whitney tests show no significant difference in males and females for global T1, T2, and T1ρ and for T1, T2 and T1ρ of most individual IVDs.
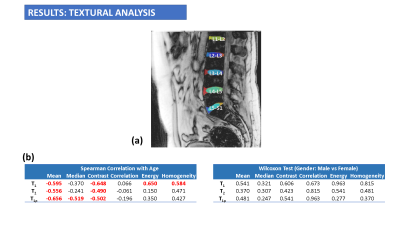
Figure 5: (a) Results from textural analysis of T1, T2 and T1ρ maps. Each IVD segment was segmented and global textural analysis was performed for all IVDs. b) Spearman correlations show significant inverse correlations between age and mean global T1, T2 and T1ρ texture. Global GLCM features on T1 maps also showed significant moderate correlations with age.