0419
Topological properties of individual gray matter morphological network effectively identify the preclinical stages of Alzheimer’s disease1School of Biological Science & Medical Engineering, Southeast University, Nanjing, China, 2Department of Radiology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China, 3Department of Medical imaging, Jingjiang People's Hospital, Jingjiang, China, 4School of Rehabilitation Medicine, Nanjing Medical University, Nanjing, China, 5Rehabilitation Medicine Department, Geriatric Hospital of Nanjing Medical University, Nanjing, China, 6Department of Radiology, The First Affiliated Hospital of Nanjing Medical University, Naning, China, 7MR Research China, GE Healthcare, Beijing, China, 8Department of Rehabilitation, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital, Suzhou, China, 9Gusu School, Nanjing Medical University, Suzhou, China, 10Rehabilitation Medicine Center, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China, 11Department of Rehabilitation, Nanjing Drum Tower Hospital Clinical College of Nanjing Medical University, Nanjing, China, 12Clinical Medicine Research Institution, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
Synopsis
Keywords: Alzheimer's Disease, Neurodegeneration
In this study, the topology of normalized individual morphological network (IMN) has been investigated for patients with subjective cognitive decline (SCD), mild cognitive impairment (MCI) and healthy controls (HCs), respectively. Significantly different topology has been revealed among SCD and MCI patients and HCs, both at global and regional level. Moreover, the alterations of the regional topological metrics, including degree centrality and nodal efficiency, at the caudate nucleus exhibited significant correlations with all clinical scales and statistically predicted the cognitive performances. Therefore, the alterations of IMN can be regarded as an effective biomarker in early detection of SCD and MCI.Introduction
Subjective cognitive decline (SCD) is regarded as the earliest alert of Alzheimer’s disease (AD)1, and mild cognitive impairment (MCI) is considered an evolutionary phase between the cognitive changes associated with aging and early stage of AD2. It is crucial to identify effective biomarkers for SCD and MCI for early diagnosis and intervention to AD. An interindividual-level analysis was proposed, based on cortical similarities in gray matter (GM) morphology within single subject and graph theory analysis3, to study single subject GM alterations in SCD, or MCI. Topological properties (32%) outperform the often-used volumetric features (9%) in explaining variance in measurements of general cognitive dysfunction4. With this method, the alterations in topological metrics of individual morphological network (IMN) can identify patients featuring fast clinical progression at predementia stages5, indicating added value of IMN topology in predicting clinical cognitive decline. However, it remains unknown whether these two preclinical stages of AD, namely SCD and MCI, can be distinguished at earlier stages according to IMN alterations, as compared to HC, and the clinical differences of these two diseases in cognitive performance can be further predicted based on the topology alterations. These two clinical questions have thus, been investigated in this study.Materials and Methods
Subjects25 SCD patients (mean: 66±7.09 years) and 29 MCI patients (mean: 70.34±7.79 years) were included in this study. 26 HCs (mean: 72.5±36.09 years), matched for sex, education years, diabetes, hypertension, lacunar infraction, and Hachinski Ischemic Scale, were also included. The written informed consent was collected from each participant. Each subject was evaluated with multiple clinical scales, with short time memory assessed by auditory-verbal learning test (AVLT), executive function assessed by Trail-Making-Test (TMT) A&B, and Animal Verbal Fluency Test (AVFT).
MRI experiment
All MRI experiments were performed on a 3T-MR scanner (Discovery 750W, GE Healthcare, USA), with a 24-channel head coil employed. 3D 1mm-isotropic high-resolution T1-weighted (T1w) MR images were acquired via a fast spoiled-gradient-echo based 3D-BRAVO sequence. The corresponding scan parameters were of: matrix size=256×256, field of view=256×256mm2, TR=8.5ms, TE=3.2ms, flip angle=12°, slice number=188, slice thickness=1mm, and bandwidth=31.25kHz. Total scan time was less than 5 minutes.
Data analysis
High-resolution T1w data were pre-processed with Computational Anatomy Toolbox and SPM12 embedded in MATLAB (R2021b). The normalized IMN was constructed from GM images based on the method proposed recently6. Topological properties of the normalized IMN, including global measurements of global efficiency (Eg), local efficiency (Eloc), normalized clustering coefficient (Gamma), clustering coefficient (Cp), normalized characteristic path length (Lambda), characteristic path length (Lp), and small world (Sigma), and regional metrics of degree centrality (DC) and nodal efficiency (Ne), were determined with GRETNA software. All statistical analyses were implemented with custom-developed scripts in MATLAB (R2021b). Analysis of covariate (ANCOVA) and subsequent post-hoc t-test were conducted to compare the differences of topological metrics of IMN among HC, SCD and MCI. Benjamin–Hochberg false discover rate (FDR) correction was utilized for multi-comparison correction to maintain FDR<0.05. Additionally, partial correlation and multiple stepwise regression were separately employed to assess the relationships between the alterations in IMN and each of the clinical scales. The effect of age was adjusted for all statistical analyses. P< 0.05 was considered statistical significance.
Results
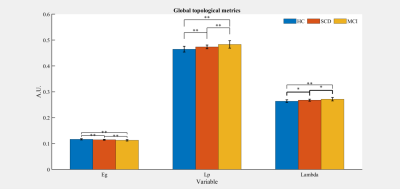
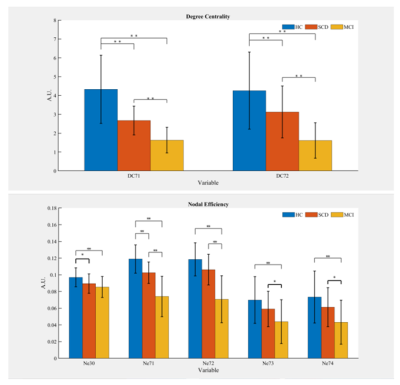
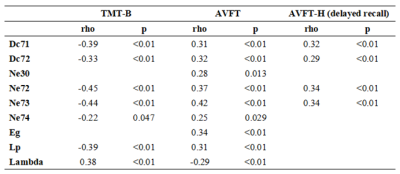
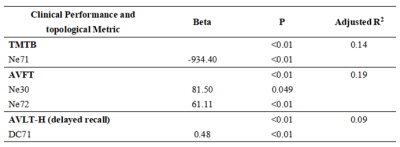
Significant differences were shown in Eg, Lp and Lambda among the three groups (all P<0.01). The post-hoc t-test revealed a sequential decrease in Eg, and a successive increase in Lp and Lambda among the three groups (all P<0.05, Fig.1). Significant differences were revealed in DC at caudate nucleus (CAU.), and Ne at CAU., right insula (INS.R), and lenticular nucleus, putamen (PUT.) among the three groups (all P<0.05). With post-hoc t-test, a consecutive decrease of DC at CAU. and a successive decrease in Ne at CAU.L was shown (all P<0.01, Fig.2). Compared to HCs, SCD and MCI patients exhibited a significant decrease in Ne at INS.R (all P<0.05, Fig.2). Additionally, significant increases in Ne at CAU.R, and PUT. were found in HCs and SCD subjects relative to MCI patients (all P<0.05, Fig.2). With partial correlation analysis, significant correlations between the regional topological measurements of DC and Ne in CAU. and each of clinical scales were exhibited (all P<0.01; Tab.1). The stepwise regression model showed that regional topology metrics of DC and Ne in CAU. could statistically predict the cognitive performances of HC, SCD and MCI subjects (all P<0.05; Tab.2). However, no global topological measurements could significantly predict the cognitive functions.Discussion and Conclusion
In this study, we systematically investigated the topology alterations, both at global and regional level, of the normalized IMNs, constructed from GM, for SCD and MCI patients, as compared to HCs. At global level, the consecutively decreased Eg and increased Lp and Lambda among HC, SCD and MCI demonstrated a sequentially decreased brain functional integration. At regional level, compared with HCs, decreased DC in CAU. and Ne in CAU., INS.R, and PUT. for SCD and MCI patients indicated a decreased functionality in these regions, being involved in many cognitive domains with executive, language, and memory. Moreover, the regional topological metrics of DC and Ne at CAU. correlate with all clinical scales, and statistically predict the cognitive performances. In conclusion, the altered IMN topology can be considered effective for early identification of SCD and MCI.Acknowledgements
No acknowledgement found.References
1. Jessen F, Amariglio R E, Buckley R F, et al. The characterisation of subjective cognitive decline[J]. The Lancet Neurology, 2020, 19(3): 271-278.
2. Petersen R C. Mild cognitive impairment: transition between aging and Alzheimer's disease[J]. Neurologia (Barcelona, Spain), 2000, 15(3): 93-101.
3. Tijms B M, Seriès P, Willshaw D J, et al. Similarity-based extraction of individual networks from gray matter MRI scans[J]. Cerebral cortex, 2012, 22(7): 1530-1541.
4. Tijms B M, Möller C, Vrenken H, et al. Single-subject grey matter graphs in Alzheimer's disease[J]. PloS one, 2013, 8(3): e58921.
5. Tijms B M, Ten Kate M, Gouw A A, et al. Gray matter networks and clinical progression in subjects with predementia Alzheimer's disease[J]. Neurobiology of aging, 2018, 61: 75-81.
6. Yu K, Wang X, Li Q, et al. Individual morphological brain network construction based on multivariate euclidean distances between brain regions[J]. Frontiers in human neuroscience, 2018, 12: 204.
Figures