0418
Diffusion abnormalities linked to brain arteriolosclerosis: An in-vivo MRI and pathology study in community-based older adults
Ana Tomash1, Mahir Tazwar1, Md Tahmid Yasar1, Shengwei Zhang2, Arnold M Evia2, David A Bennett2, Julie A Schneider2, and Konstantinos Arfanakis1,2
1Department of Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States, 2Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, IL, United States
1Department of Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States, 2Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, IL, United States
Synopsis
Keywords: Dementia, Vessels, Alzheimer's disease, Aging, Hypertension
Brain arteriolosclerosis is one of the main pathologies of cerebral small vessel disease, is common in older adults, and is associated with lower cognitive and motor function and higher odds of dementia. This work combined in-vivo MRI and pathology in a community-cohort of older adults to investigate the independent association of brain arteriolosclerosis with diffusion abnormalities in white matter. Brain arteriolosclerosis was shown to be associated with lower FA and higher trace of the diffusion tensor that extended throughout white matter, and these associations were independent of the effects of other neuropathologies or white matter hyperintensities.Introduction
Brain arteriolosclerosis is one of the main pathologies of cerebral small vessel disease and involves thickening of the vessel wall and stenosis of arterioles1. Arteriolosclerosis is common in older adults and is more severe in women2 and black older adults3. It is associated with lower cognitive and motor function4, and higher odds of dementia5. Arteriolosclerosis is strongly associated with white matter hyperintensities (WMH) in the brain6. However, the association of arteriolosclerosis with anomalies in the diffusion properties of white matter above and beyond the effects of visible WMH has not been studied. Therefore, the aim of this work was to investigate the independent association of brain arteriolosclerosis with diffusion abnormalities in white matter of community-based older adults.Methods
Participants, MRI, neuropathologyCommunity-dwelling older adults (N=175) participating in four longitudinal cohort studies of aging7,8 (Rush Memory and Aging Project, Religious Orders Study, Minority Aging Research Study, and Clinical Core of the Rush Alzheimer’s Disease Research Center) were included in this work (Fig.1). Whole brain 3D T1w MPRAGE (1×1×1mm3), T2w FLAIR (0.9×0.9×4mm3), and diffusion-weighted MRI (2×2×2mm3, b=1,000s/mm2 for 40 diffusion gradient directions, and 6 b=0s/mm2 volumes) data were acquired in-vivo on all participants using 3T MRI scanners. The intracranial space was segmented on the MRPAGE images using CAT129 and the intracranial volume was calculated. WMH were automatically segmented based on MPRAGE and FLAIR information10. The total volume of WMH was normalized with the intracranial volume, multiplied by 100 and converted by the logarithm base 10 to make it more normally distributed. TORTOISE was used on the diffusion data to correct eddy-current distortions and bulk-motion, reorient the B-matrix, calculate diffusion tensors, and generate maps of fractional anisotropy (FA) and trace of the diffusion tensor11. The FA and trace maps were transformed to the space of the IIT Human Brain Atlas v.5.012 using tensor-based registration (DR-TAMAS)13, and white matter FA and trace information was projected onto the white matter skeleton of the IIT atlas14. Participants died at an average of 3 years after in-vivo MRI (Fig.1). Following a participant’s death, the brain was extracted and underwent detailed neuropathologic evaluation by a board-certified neuropathologist (Fig.2). Severity of arteriolosclerosis was summarized in 4 levels (0-none, 1-mild, 2-moderate, and 3-severe)15. Other neuropathologies that were assessed included: Alzheimer's pathology, Lewy bodies, limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC), gross and microscopic infarcts, atherosclerosis, and cerebral amyloid angiopathy.
Statistical analysis
Linear regression was used in voxels of the white matter skeleton to test the association of FA or trace with the severity of arteriolosclerosis controlling for all other neuropathologies, WMH, demographic variables (age at MRI, sex, years of education), antemortem interval, and scanner. The statistical analyses were carried out using FSL’s PALM tool with 5,000 permutations and threshold-free cluster enhancement16. The significance level was set at p<0.05, after family-wise error rate (FWER) correction for multiple testing.
Results
Arteriolosclerosis score was associated with lower FA and higher trace of the diffusion tensor in multiple white matter regions throughout the brain (Figs.3,4). These associations were independent of other neuropathologies or the effects of WMH on diffusion properties and were present even in white matter regions that are commonly free of WMH.Discussion
This study demonstrated that brain arteriolosclerosis in older adults is linked to diffusion abnormalities that extend throughout white matter, independently of the effects of other neuropathologies or visible WMH. These findings enhance our understanding of the mechanisms through which arteriolosclerosis impacts the older adult brain. Additionally, this study suggests that by adding diffusion properties such as mean diffusivity to ARTS17, a recently introduced marker of arteriolosclerosis, we may be able to further increase its performance in predicting arteriolosclerosis in-vivo. The strengths of the present work include a) short antemortem intervals reducing the possibility of additional pathologies developing after in-vivo MRI, b) detailed neuropathology which allows for a better control for the effects of comorbid pathologies, c) a community cohort which enhances generalizability of findings, and d) good quality neuroimaging data and careful image processing.Conclusion
The present work demonstrated that brain arteriolosclerosis in older adults is linked to diffusion abnormalities that extend throughout white matter, independently of the effects of other neuropathologies or visible WMH. These findings enhance our understanding of the impact of arteriolosclerosis on the older adult brain, and may aid in improving the performance of available in-vivo markers of arteriolosclerosis such as ARTS17.Acknowledgements
This study was supported by the following grants:National Institute on Aging (NIA): R01AG064233, R01AG067482, R01AG017917, R01AG015819, RF1AG022018, R01AG056405, R01AG052200, P30AG010161, P30AG072975
National Institute of Neurological Disorders and Stroke (NINDS): UH2-UH3NS100599, UF1NS100599, R21NS076827
References
1. Blevins BL, Vinters HV, Love S, et al. Brain arteriolosclerosis. Acta Neuropathol (Berl). 2021;141(1):1-24.2. Oveisgharan S, Arvanitakis Z, Yu L, et al. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathol (Berl). 2018;136(6):887-900.
3. Barnes LL, Leurgans S, Aggarwal NT, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85(6):528-534.
4. Buchman AS, Yu L, Boyle PA, et al. Microvascular brain pathology and late-life motor impairment. Neurology. 2013;80(8):712-718.
5. Arvanitakis Z, Capuano AW, Leurgans SE, et al. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. 2016;15(9):934-943.
6. Arfanakis K, Evia AM, Leurgans SE, et al. Neuropathologic Correlates of White Matter Hyperintensities in a Community-Based Cohort of Older Adults. Flanagan M, ed. J Alzheimers Dis. 2020;73(1):333-345.
7. Bennett DA, Buchman AS, Boyle PA, et al. Religious Orders Study and Rush Memory and Aging Project. Perry G, Avila J, Moreira PI, Sorensen AA, Tabaton M, eds. J Alzheimers Dis. 2018;64(s1):S161-S189.
8. L. Barnes L, C. Shah R, T. Aggarwal N, et al. The Minority Aging Research Study: Ongoing Efforts to Obtain Brain Donation in African Americans without Dementia. Curr Alzheimer Res. 2012;9(6):734-745.
9. Farokhian F, Beheshti I, Sone D, et al. Comparing CAT12 and VBM8 for Detecting Brain Morphological Abnormalities in Temporal Lobe Epilepsy. Front Neurol. 2017;8:428.
10. Li H, Jiang G, Zhang J, et al. Fully convolutional network ensembles for white matter hyperintensities segmentation in MR images. NeuroImage. 2018;183:650-665.
11. C. Pierpaoli, L. Walker, M. O. Irfanoglu, et al. TORTOISE: an integrated software package for processing of diffusion MRI data, ISMRM 18th annual meeting, Stockholm, Sweden, 2010, abstract #1597.
12. Zhang S, Arfanakis K. Evaluation of standardized and study-specific diffusion tensor imaging templates of the adult human brain: Template characteristics, spatial normalization accuracy, and detection of small inter-group FA differences. NeuroImage. 2018;172:40-50.
13. Irfanoglu MO, Nayak A, Jenkins J, et al. DR-TAMAS: Diffeomorphic Registration for Tensor Accurate Alignment of Anatomical Structures. NeuroImage. 2016;132:439-454.
14. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487-1505.
15. Buchman AS, Leurgans SE, Nag S, et al. Cerebrovascular Disease Pathology and Parkinsonian Signs in Old Age. Stroke. 2011;42(11):3183-3189.
16. Winkler AM, Ridgway GR, Webster MA, et al. Permutation inference for the general linear model. NeuroImage. 2014;92:381-397.
17. Makkinejad N, Evia AM, Tamhane AA, et al. ARTS: A novel In-vivo classifier of arteriolosclerosis for the older adult brain. NeuroImage Clin. 2021;31:102768.
Figures
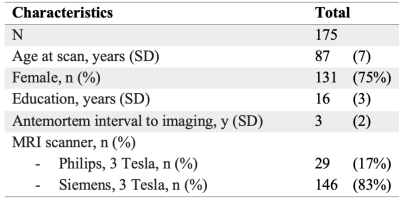
Figure 1: Demographic characteristics
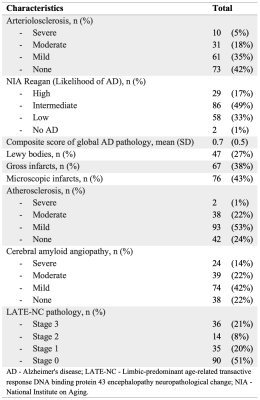
Figure 2: Neuropathological characteristics
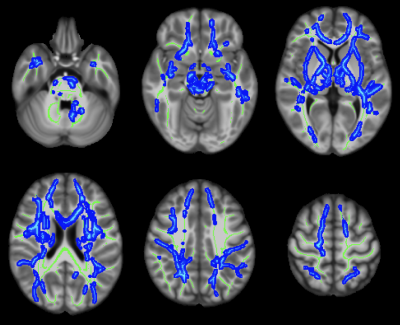
Figure 3: Axial color maps (blue scale) of white matter regions with a negative association of fractional anisotropy (FA) with arteriolosclerosis, overlaid on the white matter skeleton (in green).
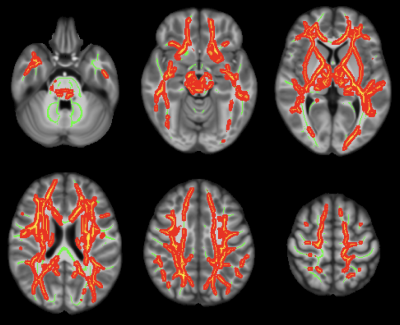
Figure 4: Axial color maps (red-yellow scale) of white matter regions with a positive association of the trace of the diffusion tensor with arteriolosclerosis, overlaid on the white matter skeleton (in green).
DOI: https://doi.org/10.58530/2023/0418