0411
Vascular-water-exchange imaging to detect blood-brain barrier breakdown in Alzheimer’s disease without contrast agent1College of Biomedical Engineering and Instrument Science, Zhejiang University, Hangzhou, China, 2School of Medicine, Zhejiang University, Hangzhou, China, 3National Clinical Research Center for Neurological Diseases, Beijing Tiantan Hospital, Capital Medical University, Beijing, China, 4Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China, 5MR Collaboration, Siemens Healthcare, Shanghai, China
Synopsis
Keywords: Alzheimer's Disease, Alzheimer's Disease, Blood-brain barrier
Blood-brain-barrier (BBB) impairment is an important pathophysiological process in Alzheimer’s disease (AD). However, most neuroimaging methods assessing BBB function require contrast agent injection, limiting the methods’ application. Vascular-water-exchange imaging (VEXI), a version of filter-exchange imaging (FEXI), is a contrast-agent-free method assessing BBB permeability to water. We quantitatively measured BBB permeability using VEXI in normal subjects, mild cognitive impairment patients, and AD patients and found BBB breakdown occurred specifically in the hippocampus, worsening with disease progression. In addition, BBB permeability to water showed a significant correlation with cognitive impairment. Therefore, VEXI might be a potential contrast-agent-free neuroimaging method.
Introduction
Increasing evidence has shown that blood-brain barrier (BBB) impairment contributes to Alzheimer’s disease (AD) pathophysiology 1,2 and might be a potential biomarker for early AD diagnosis. Studies using these neuroimaging methods, including positron emission tomography (PET) and magnetic resonance imaging (MRI), have shown increased BBB permeability in different brain regions in AD patients3,4. However, most conventionally used neuroimaging methods assessing BBB permeability in AD require the intravenous administration of contrast agents (or radioactive tracers). Therefore, a non-invasive and contrast-agent-free neuroimaging method to characterize BBB permeability is still desirable. Vascular-water-exchange imaging (VEXI) is a contrast-agent-free method assessing the speed of water exchange across the BBB based on filter-exchange imaging (FEXI)5.In this study, we aimed to explore the feasibility of VEXI to assess BBB permeability to water molecules in detecting BBB breakdown in AD.Methods
VEXI characterizes the BBB permeability to water molecules. It is a specific diffusion-based FEXI adapted for measuring water exchange across the BBB; details on the data acquisition and analysis can be found in [5]. In VEXI, the apparent exchange rate across BBB (AXRBBB) is considered the quantitative parameter characterizing BBB permeability to water molecules.Eleven AD patients (AD group), fourteen mild cognitive impairment patients (MCI group), and 27 age-matched normal subjects (NC group) were scanned on a 3T MRI (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). VEXI data were acquired with one b-value in the filter block (bf = 250 s/mm2) and two b-values in the detection block (bd = 0 s/mm2 with six repetitions and 250 s/mm2 with ten repetitions). Imaging was repeated with three mixing times (tm): 25, 200, and 400 ms. VEXI was also acquired with bf = 0 s/mm2 and the shortest tm (25 ms). Finally, the voxel size was 3.0 ´ 3.0 ´ 5.0 mm3, and the number of slices was 20.
All VEXI data underwent pre-processing, including motion and eddy current distortion correction in TORTOISE, and the model fitting was performed with an in-house program developed in MATLAB (2018B, The MathWorks Inc., Natick, Massachusetts, USA). Then, we generated a study-specific template with the T1-weighted images of all subjects in the NC group; parameter maps (AXRBBB, ADC and clip_image002.png">) were then iteratively aligned to the template. Next, eight region-of-interest (ROI) masks were extracted from Brainnetome Atlas and JHU white matter atlas, as shown in Figure 1. Finally, the parametric metrics in each ROI were calculated as the median of all voxels in this ROI.
Results and Discussion
The average ADC maps of all subjects in the MCI and NC groups and representative ADC (tm) curves of the hippocampus are shown in Figures 2A and 2B. Further quantitative modeling revealed that the AXRBBB of the hippocampus in the MCI group (group-averaged AXRBBB = 1.63 s-1) was significantly larger than in the NC group (AXRBBB = 1.21 s-1, p = 0.042, Figure 2C), and this occurred specifically in the hippocampus, consistent with previous reports6,7.Interestingly, we found that as the disease progressed, the BBB breakdown worsened, extending to more brain regions. As shown in Figure 3, we found increased AXRBBB in the hippocampus along the trajectory from MCI to AD. Further ANOVA analyses revealed that the thalamus and orbital frontal cortex (OFC) also showed significantly increasing AXRBBB (increased by 59.4% and 130.0%, respectively) from the MCI to AD groups (p = 0.0006 and p = 0.0001, respectively, Tukey’s post hoc test after ANOVA analysis) other than the hippocampus. Although the BBB breakdown of the thalamus and OFC has not been reported in recent DCE-MRI studies3,6,7, a previous study on the AD rat model found that water exchange across the BBB showed its breakdown in several brain regions (including the hippocampus, thalamus, and cortex). However, Ktrans failed to detect any BBB breakdown8. This showed that water exchange across the BBB may be a sensitive biomarker.
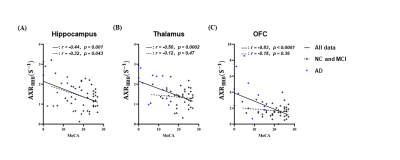
Linear regression was used to assess potential correlations between BBB impairment and cognitive dysfunction. In Figure 4, we show that although the AXRBBB of all three brain regions demonstrated significant and negative correlations with the MoCA score in all subjects (r = -0.44, p = 0.001 in the hippocampus, r = -0.50, p = 0.0002 in the thalamus and r = -0.53, p < 0.0001 in the OFC), only in the hippocampus did this correlation remain significant in the absence of the AD group (r = -0.32, p = 0.043), suggesting that the worse cognitive function is associated with more extensive hippocampal BBB impairment. In contrast, BBB impairment is related to cognitive dysfunction in the thalamus and OFC only in the late stages of the disease.
Conclusion
We found BBB breakdown occurred specifically in the hippocampus from the NC to the MCI groups and worsened from the MCI to the AD groups, extending to the thalamus and OFC regions. Furthermore, the BBB permeability to water detected by VEXI showed a significant correlation with cognitive dysfunction. Our results have demonstrated the feasibility of VEXI for detecting BBB breakdown in AD and of VEXI as a potential contrast agent-free neuroimaging method in the long-term studies of a large population.Acknowledgements
No acknowledgement found.References
1. Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease[J]. Neuron, 2017, 96(1): 17-42.
2. Sweeney M D, Sagare A P, Zlokovic B V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders[J]. Nature Reviews Neurology, 2018, 14(3): 133-150.
3. Van De Haar H J, Jansen J F A, Jeukens C R, et al. Subtle blood‐brain barrier leakage rate and spatial extent: considerations for dynamic contrast‐enhanced MRI[J]. Medical Physics, 2017, 44(8): 4112-4125.
4. Wang H, Golob E J, Su M Y. Vascular volume and blood‐brain barrier permeability measured by dynamic contrast enhanced MRI in hippocampus and cerebellum of patients with MCI and normal controls[J]. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine, 2006, 24(3): 695-700.
5. Bai R, Li Z, Sun C, et al. Feasibility of filter-exchange imaging (FEXI) in measuring different exchange processes in human brain[J]. NeuroImage, 2020, 219: 117039.
6. Montagne A, Barnes S R, Sweeney M D, et al. Blood-brain barrier breakdown in the aging human hippocampus[J]. Neuron, 2015, 85(2): 296-302.
7. Nation D A, Sweeney M D, Montagne A, et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction[J]. Nature medicine, 2019, 25(2): 270-276.
8. Dickie B R, Parker G J M, Parkes L M. Measuring water exchange across the blood-brain barrier using MRI[J]. Progress in Nuclear Magnetic Resonance Spectroscopy, 2020, 116: 19-39.
Figures
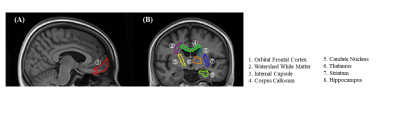
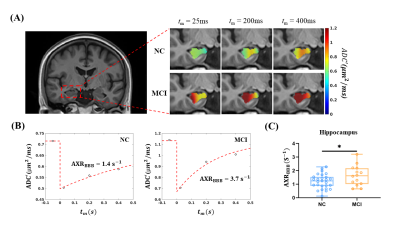
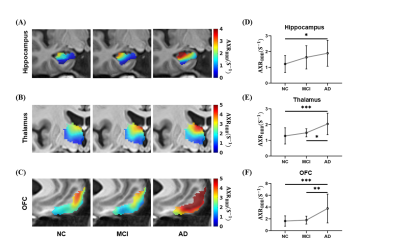
Figure 3. VEXI showed BBB breakdown with disease progression. (A-C) Averaged AXRBBB maps of all subjects in the three groups in the hippocampus, thalamus, and OFC in the template space. Statistical comparison of AXRBBB values in the hippocampus (D), thalamus (E), and OFC (F) among the NC, MCI, and AD groups. p, the significance by ANOVA followed by Tukey’s post hoc tests. * p < 0.05, ** p < 0.01, *** p < 0.001.