0409
Thalamic nuclei changes in early vs. late onset Alzheimer’s Disease1Alzheimer’s Disease and Other Cognitive Disorders Unit, Neurology Service, Hospital Clínic of Barcelona, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain, 2Escuela de Psicología, Universidad de los Andes, Santiago, Chile, 3University of East Anglia, Norwich, United Kingdom, 4Radiology, University of Massachusetts Chan Medical School, Worcester, MA, United States
Synopsis
Keywords: Alzheimer's Disease, Segmentation
Age at symptom onset distinctly affects thalamic nuclei alongside AD. While Early Onset AD showed significant anteroventral (AV) nucleus changes associated with increasing tau pathology, Late onset AD AV nucleus was associated with Aβ42 levels but no significant volume changes were found.Introduction
Alzheimer’s disease (AD) is the most common cause of dementia worldwide. Most commonly associated with impaired episodic memory, AD has been characterized mainly as a ‘hippocampal dementia’ (Craig et al., 2011). Emerging evidence shows that the thalamus is also an important hub in the clinical symptomatology of the disease, with the anterior, laterodorsal, and the mediodorsal thalamic nuclei (‘limbic thalamus’) (Taber et al., 2004), been described as especially vulnerable (Aggleton et al., 2016). While whole thalamic atrophy has been reported in AD, there are only handful of studies that have examined thalamic nuclei in AD (Bernstein et al., 2021; Iglesias et al., 2018; Low et al., 2019). This is largely due to the paucity of robust thalamic nuclei segmentation methods for resolving small structures such as anteroventral, centromedian, and lateral/medial geniculate nuclei. In this work, we looked at thalamic atrophy in early-onset AD ((EAOD) 65 years < age at symptom onset) and late-onset AD ((LOAD) 65 years > age at symptom onset)) compared to healthy controls (HC) using a recently developed cutting-edge deep learning based thalamic segmentation method.Methods
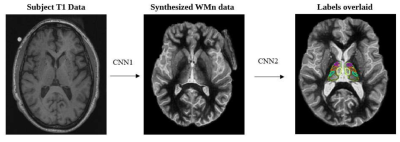
Thalamus Optimized Multi Atlas Segmentation (THOMAS) (Su et al., 2019) has been shown to produce robust and accurate segmentation of thalamic nuclei on white-matter nulled MPRAGE data, where intrathalamic and outer thalamic boundaries are delineated clearly. However, this special sequence is not part of most clinical protocols. The recently developed deep learning variant of THOMAS (THOMAS-DL) addresses this problem by first synthesizing WMn data from T1 data and then segmenting the synthetic WMn data. Both synthesis and segmentation are accomplished using 2.5D U-net architectures (Umapathy et al., 2021) as shown in Figure 1.THOMAS-DL was shown to be more sensitive and accurate than direct T1-based segmentation (Umapathy et al., 2021). In this work, THOMAS-DL was used to parcellate 11 thalamic nuclei per hemisphere from T1-weighted MRI in 88 biomarker confirmed AD patients (49 EOAD and 39 LOAD) and 58 healthy controls (HC). The output of each lateralized nucleus was summed to get the overall volume. Nuclei volumes were compared among groups using MANCOVA with age and intracranial volume as covariates of interest followed by Sidak’s post-hoc test. Pearson’s correlation coefficient between thalamic nuclear volume (after adjusting for age and ICV) with neuropsychological scores and CSF-biomarkers were computed to assess the association between thalamic nuclear volume changes and cognition and with CSF-biomarkers values.Results
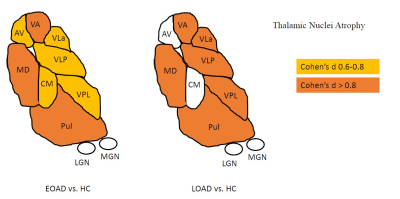
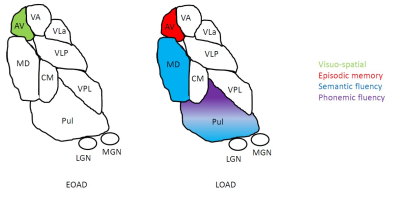
Overall, EOAD and LOAD showed significant volume reduction in the ventral anterior (VA), ventral lateral anterior (VLa), ventral lateral posterior (VLp), ventral posterior lateral (VPL), pulvinar and mediodorsal (MD) nuclei compared to HC. Reduced anteroventral (AV) and centromedian (CM) nuclei were only found in EOAD when compared to HC (Figure 2). No significant differences were found when comparing EOAD to LOAD. In EOAD, the AV nucleus positively correlated with visuospatial abilities. For LOAD, the AV nucleus showed positive correlations with episodic memory, the pulvinar and MD nucleus correlated with semantic fluency, while only the Pulvinar positively correlated with phonemic fluency. These are depicted in Figure 3. For CSF-biomarkers, EOAD AV nucleus showed negative correlations with total tau (r = -0.319, p ≤ 0.05), while LOAD AV nucleus showed negative correlations with Aβ42 (r = -0.360, p ≤ 0.05).Conclusion
Overall, EOAD and LOAD showed significantly reduced thalamic volume compared to HC, which were significantly associated with cognition and CSF-biomarkers. Reduced AV nucleus was only significant in EOAD (compared to HC). This is surprising as the AV nucleus has been characterized as critical for memory and visuospatial abilities and associated as a function of increasing cognitive impairment (Bernstein et al., 2021). This could be partially explained as younger age at symptom onset has been related with greater AD pathological burden, with stronger tau deposition associated with earlier AD symptom manifestation and faster cognitive decline (Frontzkowski et al., 2022). In this study, we showed tau pathology associated with the AV nucleus only for EOAD, while LOAD AV nucleus was associated with Aβ42. Overall, our results are in line with more recent studies showing thalamic nuclei volume changes in AD and establishes THOMAS-DL as a sensitive tool in investigating thalamic pathology in AD.Acknowledgements
To the patients and the clinical team that collaborate in the recruitment and assessment.References
Aggleton, J. P., Pralus, A., Nelson, A. J., & Hornberger, M. (2016, Jul). Thalamic pathology and memory loss in early Alzheimer's disease: moving the focus from the medial temporal lobe to Papez circuit. Brain, 139(Pt 7), 1877-1890. https://doi.org/10.1093/brain/aww083
Bernstein, A. S., Rapcsak, S. Z., Hornberger, M., Saranathan, M., & Alzheimer's Disease Neuroimaging, I. (2021). Structural Changes in Thalamic Nuclei Across Prodromal and Clinical Alzheimer's Disease. J Alzheimers Dis, 82(1), 361-371. https://doi.org/10.3233/JAD-201583 Craig, L. A., Hong, N. S., & McDonald, R. J. (2011, May). Revisiting the cholinergic hypothesis in the development of Alzheimer's disease. Neurosci Biobehav Rev, 35(6), 1397-1409. https://doi.org/10.1016/j.neubiorev.2011.03.001
Frontzkowski, L., Ewers, M., Brendel, M., Biel, D., Ossenkoppele, R., Hager, P., Steward, A., Dewenter, A., Romer, S., Rubinski, A., Buerger, K., Janowitz, D., Binette, A. P., Smith, R., Strandberg, O., Carlgren, N. M., Dichgans, M., Hansson, O., & Franzmeier, N. (2022, Aug 20). Earlier Alzheimer's disease onset is associated with tau pathology in brain hub regions and facilitated tau spreading. Nat Commun, 13(1), 4899. https://doi.org/10.1038/s41467-022-32592-7
Iglesias, J. E., Insausti, R., Lerma-Usabiaga, G., Bocchetta, M., Van Leemput, K., Greve, D. N., van der Kouwe, A., Alzheimer's Disease Neuroimaging, I., Fischl, B., Caballero-Gaudes, C., & Paz-Alonso, P. M. (2018, Dec). A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. Neuroimage, 183, 314-326. https://doi.org/10.1016/j.neuroimage.2018.08.012
Low, A., Mak, E., Malpetti, M., Chouliaras, L., Nicastro, N., Su, L., Holland, N., Rittman, T., Rodriguez, P. V., Passamonti, L., Bevan-Jones, W. R., Jones, P. S., Rowe, J. B., & O'Brien, J. T. (2019, Dec). Asymmetrical atrophy of thalamic subnuclei in Alzheimer's disease and amyloid-positive mild cognitive impairment is associated with key clinical features. Alzheimers Dement (Amst), 11, 690-699. https://doi.org/10.1016/j.dadm.2019.08.001
Su, J. H., Thomas, F. T., Kasoff, W. S., Tourdias, T., Choi, E. Y., Rutt, B. K., & Saranathan, M. (2019, Jul 1). Thalamus Optimized Multi Atlas Segmentation (THOMAS): fast, fully automated segmentation of thalamic nuclei from structural MRI. Neuroimage, 194, 272-282. https://doi.org/10.1016/j.neuroimage.2019.03.021
Taber, K. H., Wen, C., Khan, A., & Hurley, R. A. (2004, Spring). The limbic thalamus. J Neuropsychiatry Clin Neurosci, 16(2), 127-132. https://doi.org/10.1176/jnp.16.2.127
Umapathy, L., Keerthivasan, M. B., Zahr, N. M., Bilgin, A., & Saranathan, M. (2021). A Contrast Synthesized Thalamic Nuclei Segmentation Scheme using Convolutional Neural Networks. NEUROINFORMATICS.
Figures