0373
Balanced Steady-State Free Precession and Radial Sampling for Arterial Spin Labeled Perfusion Imaging1Gordon Center for Medical Imaging, Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States
Synopsis
Keywords: Arterial spin labelling, Perfusion, Brain
Arterial spin labeling (ASL) is a non-invasive MRI technique that allows to quantitatively measure cerebral blood flow. However, the major limitation of ASL is in the intrinsically low signal-to-noise ratio (SNR). Balanced steady-state free precession (bSSFP) sequence has been proposed to mitigate this limitation, however, bSSFP is sensitive to off-resonance effects. ASL perfusion imaging with bSSFP can be sensitive to effects from motion and flow when Cartesian sampling scheme is used. This work proposes and investigates radial sampling scheme for ASL with bSSFP to allow perfusion imaging with relatively high SNR and robustness to motion and off-resonance effects.Introduction
Arterial spin labeling (ASL) is a non-invasive MRI technique that allows to quantitatively measure cerebral blood flow. Despite the advantages and usefulness, the major disadvantage of ASL is in the intrinsically low signal-to-noise ratio (SNR) that it can provide. Balanced steady-state free precession (bSSFP), a sequence that is known to provide the highest SNR per unit time1, has been proposed for ASL perfusion imaging2,3 with great potential to mitigate this limitation. However, bSSFP is sensitive to off-resonance effects which results in banding artifacts in regions with severe B0 inhomogeneity1. Also, ASL perfusion imaging with bSSFP can be sensitive to effects from motion and flow when Cartesian sampling scheme is used3. In this work, we propose radial sampling scheme for ASL perfusion imaging with bSSFP. The usefulness of radial sampling scheme is demonstrated for ASL perfusion imaging with bSSFP via in vivo studies from healthy volunteers.Methods
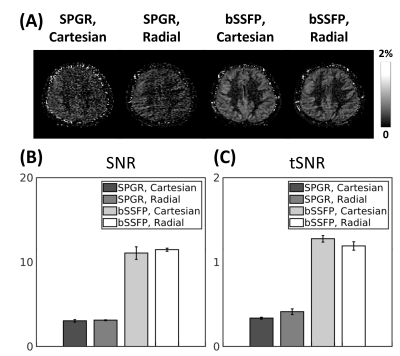
In vivo experiments were performed using a 3T MR scanner (MAGNETOM Prisma, Siemens Healthineers, Erlangen, Germany) with body-coil for signal transmission and 32-channel head coil for signal reception. The study protocol was approved by our local Institutional Review Board. ASL perfusion imaging was performed using pseudo-continuous ASL (pCASL) labeling scheme4,5 and background suppression (BS)6 with the following parameters: labeling duration=1.5s; post-labeling delay (PLD)=1.5s; labeling plane= 8.5cm inferior to the anterior commissure-posterior commissure (AC-PC) line; Hanning window-shaped RF pulse; B1 average=1.63$$$\mu$$$T; average labeling gradient=1.0mT/m; slice-selective labeling gradient=6.0mT/m; and unbalanced tagging scheme. The general imaging parameters for bSSFP were: TR/TE=4∕2 ms; flip angle=30$$$^{\circ}$$$; receiver bandwidth=592Hz/pixel; and RF phase increment ($$$\triangle\phi$$$)$$$=\pi/2$$$, unless otherwise noted. For all studies, a single scan without pCASL labeling and BS was additionally acquired for quantification of the perfusion signal.A 2D perfusion imaging study was performed to compare the differences between bSSFP and spoiled gradient echo (SPGR) sequences with Cartesian and radial sampling schemes. The common imaging parameters were: field-of-view (FOV)=240$$$\times$$$240 and 180$$$\times$$$180mm2 for Cartesian and radial sampling schemes, respectively; resolution=1.875$$$\times$$$1.875mm2; slice thickness=5mm; acquisition time per imaging session=3.8min; and number of imaging sessions=3. The imaging parameters specific to SPGR were: TR/TE=4/1.7ms; flip angle=10$$$^{\circ}$$$; and receiver bandwidth=1400Hz/pixel.
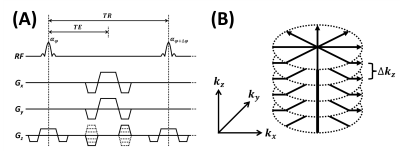
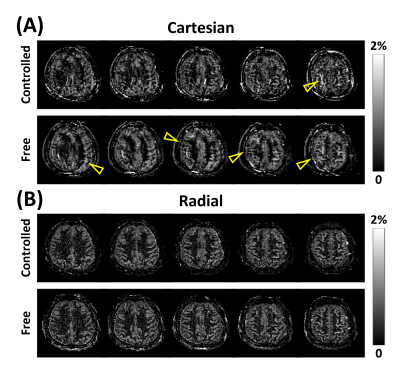
To investigate the performance with respect to respiratory-related motion, a 3D perfusion imaging study was performed using segmented 3D acquisitions of bSSFP following Cartesian and stack-of-stars sampling trajectory (Fig.1), with and without controlled breathing strategy7. In the case of controlled breathing, subjects were instructed to breathe voluntarily while holding their breath at times of image acquisitions. The remaining imaging parameters of bSSFP were: FOV=240$$$\times$$$240$$$\times$$$120 and 180$$$\times$$$180$$$\times$$$120mm3 for Cartesian and radial sampling schemes, respectively; resolution = 1.875$$$\times$$$1.875$$$\times$$$5mm3; and acquisition time= 4.6min.
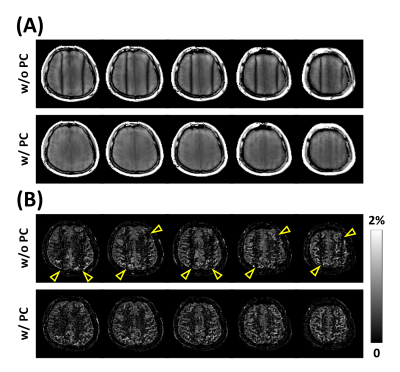
To investigate the feasibility and effect of combining with phase-cycling technique, a 3D perfusion imaging study was performed using segmented 3D acquisitions of bSSFP following stack-of-stars sampling trajectory (Fig.1), with and without phase-cycling. Additional linear gradient of 100 $$$\mu$$$T/m was applied along the right-left direction after shimming to induce banding artifacts along the direction. The remaining imaging parameters of bSSFP were: FOV=180$$$\times$$$180$$$\times$$$60mm3; resolution=1.875$$$\times$$$1.875$$$\times$$$5mm3; and acquisition time=4.6min. In case of phase-cycling, phase-cycled images were acquired with $$$\triangle\phi=0$$$,$$$\pi/2$$$,$$$\pi$$$,$$$3\pi/2$$$ and were combined using a nonlinear averaging method8.
To investigate the feasibility of accelerating the imaging time, additional 3D perfusion imaging study was performed using segmented 3D acquisition of bSSFP following stack-of-stars sampling trajectory (Fig.1). The remaining imaging parameters of bSSFP were: FOV=180$$$\times$$$180$$$\times$$$120mm3; resolution = 1.875$$$\times$$$1.875$$$\times$$$5mm3; and acquisition time=2.3min and 1.15 min for full and under-sampled cases, respectively. In case of under-sampling, data was prospectively under-sampled with reduction factor (R) of 2. Image reconstruction was performed by solving the following optimization problem:
$$ \arg\min_{x}\parallel\Omega\mathcal{F}Sx-y\parallel^2_2+\lambda TV(x)$$
where $$$x$$$ denotes the reconstructed image, $$$\Omega$$$ denotes the sampling mask, $$$\mathcal{F}$$$ denotes the spatial Fourier transform, $$$S$$$ denotes the coil sensitivity, $$$y$$$ denotes the measured k-space data, $$$\lambda$$$ denotes the regularization parameter, and $$$TV(\cdot)$$$ denotes total variation regularization9. Non-uniform fast Fourier transform (NuFFT)10 was used for $$$\mathcal{F}$$$. The optimization problem was solved using SENSE-based reconstruction algorithm11,12 with alternating direction method of multipliers (ADMM)13.
Results and Discussion
ASL with bSSFP showed higher spatial and temporal SNRs of the perfusion signal compared to those with SPGR (Fig.2). Cartesian and radial sampling schemes showed similar performance in terms of spatial and temporal SNRs of the perfusion signal, regardless of the imaging sequence (Fig.2). ASL perfusion imaging with bSSFP and Cartesian sampling scheme was sensitive to effects from respiratory-related motion, resulting in artifacts in the perfusion-weighted images (Fig.3a). In comparison, ASL perfusion imaging with bSSFP and radial sampling scheme was robust to these effects (Fig.3b). Banding artifacts were observed in the images from the single RF phase incremented bSSFP acquisitions when additional linear gradient was applied after shimming (Fig.4). Effects from these artifacts were significantly reduced when phase-cycling technique was used (Fig.4). Perfusion-weighted images were successfully reconstructed from under-sampled acquisition, demonstrating the feasibility of accelerating the imaging time for pCASL-bSSFP with radial sampling (Fig.5).Conclusion
ASL with bSSFP and radial sampling scheme allows perfusion imaging with relatively high SNR and robustness to motion. Additional robustness to off-resonance effects can be achieved by employing phase-cycling technique. Imaging time of the proposed method can be accelerated with the help of under-sampling and image reconstruction.Acknowledgements
This work was supported in part by the National Institutes of Health (P41EB022544, R01CA165221, R01HL137230, T32EB013180, and K01EB030045).References
1. Scheffler, Klaus, and Stefan Lehnhardt. "Principles and applications of balanced SSFP techniques." European radiology 13.11 (2003): 2409-2418.
2. Park, Sung-Hong, Danny JJ Wang, and Timothy Q. Duong. "Balanced steady state free precession for arterial spin labeling MRI: initial experience for blood flow mapping in human brain, retina, and kidney." Magnetic resonance imaging 31.7 (2013): 1044-1050.
3. Han, Paul Kyu, et al. "Whole‐brain perfusion imaging with balanced steady‐state free precession arterial spin labeling." NMR in biomedicine 29.3 (2016): 264-274.
4. Wu, Wen‐Chau, et al. "A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 58.5 (2007): 1020-1027.
5. Dai, Weiying, et al. "Continuous flow‐driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 60.6 (2008): 1488-1497.
6. Maleki, Nasim, Weiying Dai, and David C. Alsop. "Optimization of background suppression for arterial spin labeling perfusion imaging." Magnetic Resonance Materials in Physics, Biology and Medicine 25.2 (2012): 127-133.
7. Robson, Philip M., et al. "Strategies for reducing respiratory motion artifacts in renal perfusion imaging with arterial spin labeling." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 61.6 (2009): 1374-1387.
8. Elliott, Andrew M., et al. "Nonlinear averaging reconstruction method for phase-cycle SSFP." Magnetic resonance imaging 25.3 (2007): 359-364.
9. Chambolle, Antonin. "An algorithm for total variation minimization and applications." Journal of Mathematical imaging and vision 20.1 (2004): 89-97.
10. Fessler, Jeffrey A., and Bradley P. Sutton. "Nonuniform fast Fourier transforms using min-max interpolation." IEEE transactions on signal processing 51.2 (2003): 560-574.
11. Pruessmann, Klaas P., et al. "SENSE: sensitivity encoding for fast MRI." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 42.5 (1999): 952-962.
12. Pruessmann, Klaas P., et al. "Advances in sensitivity encoding with arbitrary k‐space trajectories." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 46.4 (2001): 638-651.
13. Boyd, Stephen, et al. "Distributed optimization and statistical learning via the alternating direction method of multipliers." Foundations and Trends® in Machine learning 3.1 (2011): 1-122.
Figures