0372
Hybrid Adiabatic Pulse with asYmmetry (HAPY): an asymmetric adiabatic pulse with an application in pulsed ASL at 7T1Department of Biomedical Engineering, University of Melbourne, Parkville, Australia, 2Melbourne Brain Centre Imaging Unit, University of Melbourne, Parkville, Australia, 3MR Research Collaborations, Siemens Healthcare Pty Ltd, Melbourne, Australia, 4MR Research Collaborations, Siemens Healthcare Pty Ltd, Sydney, Australia, 5MR Research Collaborations, Siemens Healthcare Pty Ltd, Brisbane, Australia
Synopsis
Keywords: Arterial spin labelling, Arterial spin labelling, Pulsed ASL
The increased power deposition and inhomogeneity of $$$\Delta{B_0}$$$ and $$$B_1^+$$$ fields limit the application of ASL at 7T. A new type of asymmetric adiabatic pulses, Hybrid Adiabatic Pulses with asYmmetry (HAPY) along with its optimisation scheme, is proposed in this study. In simulation and phantom experiments, the proposed HAPY pulse demonstrates RF labelling pulse energy reduction and high labelling efficiency under challenging $$$\Delta{B_0}$$$ and $$$B_1^+$$$ conditions. In vivo experiments show the successful application of PICORE-PASL with HAPY labelling pulses in 7T ASL with increased temporal resolution.Introduction
ASL at ultra-high field is known to benefit from the intrinsic SNR gain and prolonged relaxation time.1-4 Despite these advantages, it remains technically challenging to fully utilise the theoretical merits of increased field strength in ASL applications. Firstly, the increased power deposition is a limiting factor for practical ASL protocols.5 Secondly, $$$B_0$$$ and $$$B_1^+$$$ field inhomogeneity reduces labelling efficiency.5,6 To overcome these challenges, adiabatic inversion pulses, including Hyperbolic Secant (HS), Gradient-modulated Offset Independent Adiabaticity (GOIA), and Frequency Offset Corrected Inversion (FOCI) pulses, have been employed in PASL.7-13An asymmetric labelling pulse was used previously14 for PICORE-PASL at 1.5T, which demonstrated that the sharpness of the proximal edge of the labelling slab was not critical to providing robust perfusion signal. In this study, we hypothesised that asymmetric adiabatic pulses would provide reduced power deposition without affecting the labelling efficiency for 7T PASL. We tested this hypothesis by proposing a present a new class of asymmetric adiabatic pulses, Hybrid Adiabatic Pulse with asYmmetry (HAPY), and its optimisation strategy and applications for PICORE-PASL at 7T.
Methods
Pulse designHAPY is an asymmetric selective adiabatic inversion pulse with tailored profiles on the critical and non-critical edges (denoted as left (L) and right (R) without loss of generality). It can be derived from the GOIA pulses, by modifying the original GOIA formulation.8
The normalised HAPY amplitude modulation function,
$$F_1(t)=\begin{cases}F_{1L}(t)=\text{sech}(\beta_{L}T_L(t))\\F_{1R}(t)=\text{sech}(\beta_R T_R(t)),\end{cases}$$
and gradient modulation function,
$$F_3(t)=\begin{cases}F_{3L}(t)=1-f_L\text{sech}(\beta_L T_L(t))\\\frac{1-f_L}{1-f_R}F_{3R}(t)=\frac{1-f_L}{1-f_R}(1-f_R\text{sech}(\beta_{R}T_R(t)),\end{cases}$$
are defined over a pulse duration of $$$D$$$ (ms), where $$$f_L$$$, $$$f_R$$$ determine the minimum values of $$$F_{3L}(t)$$$ and $$$F_{3R}(t)$$$. In the above equations, $$$T_L(t)$$$ and $$$T_R(t)$$$ are monotonically increasing time-driving functions, akin to Hurley et al.’s study,15 with the following form:
$$T_L(t)=\frac{{\tau_1}_L{t^5}+{\tau_2}_L{t^3}+t}{{\tau_1}_L+{\tau_2}_L+1},\quad -1{\leq}t\leq\frac{2D_L}{D}-1$$ $$T_R(t)=\frac{{\tau_1}_R{t^5}+{\tau_2}_R{t^3}+t}{{\tau_1}_R+{\tau_2}_R+1},\quad\frac{2D_L}{D}-1<t\leq{1},$$
in which $$$D_L$$$ is the pulse duration of left half of HAPY. The frequency modulation function, $$$F_2(t)$$$, is obtained based on $$$F_1(t)$$$ and $$$F_3(t)$$$ as proposed in Tannùs and Garwood’s work.8
Optimised GOIA and HAPY pulses were obtained via a particle swarm algorithm by iteratively minimising the loss function
$$\mathcal{L}=\sum_{\Delta{B_0},B_1^+}\left(\mathcal{L}_\text{inv}+\lambda_1\mathcal{L}_\text{smooth} \right)+\lambda_2\mathcal{L}_\text{SAR},$$ where $$$L_\text{inv}$$$ is the MSE between the simulated and ideal inversion profiles over a width of $$$2\Delta x$$$ ($$$\Delta x$$$: desired inversion thickness) over three segments (Figure 1a); $$$L_\text{smooth}$$$ is the total variation of the simulated inversion profile; $$$L_\text{SAR} = \int_0^D F_1^2(t) dt$$$ penalises pulse energy. Realistic $$$\Delta{B_0}$$$ and $$$B_1^+$$$ conditions were selected at the 10th percentile contour of the joint ($$$\Delta{B_0}$$$,$$$B_1^+$$$) histogram (Figure 1b) based on co-registered $$$\Delta{B_0}$$$ and $$$B_1^+$$$ maps of four healthy volunteers.
A two-step optimisation process was performed by firstly optimising the base GOIA pulse with sharp transition, which was subsequently used as the critical side of HAPY, and secondly optimising the non-critical side of HAPY with wider transition edge but greatly reduced energy (Figure 2).
Simulation
The optimised GOIA and HAPY pulses were compared in Bloch equation simulation with HS-3 and trFOCI pulses under six conditions: $$$({B_1^+}(\mu\text{T}),\Delta{B_0}(\text{Hz}))\in\{(5,0),(5,-340),(5,210),(10,0),(10,-340),(10,210)\}$$$. HS-3 and trFOCI were rescaled to match the pulse energy as the GOIA pulse while HAPY and GOIA had the same peak inversion voltages.
Experiments
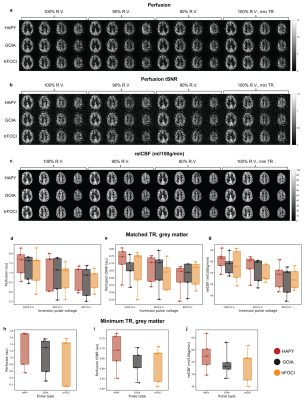
Phantom and in vivo scans were performed on an investigational 7T whole-body MRI scanner (MAGNETOM 7T plus, Siemens Healthcare, Erlangen, Germany) using a 1Tx-32Rx head coil (Nova Medical Inc., Wilmington, MA, USA). Inversion profiles of GOIA, HAPY, trFOCI, and HS-3 pulses were compared using a phantom experiment; a custom IR-FLASH sequence with inversion applied along the read-out direction was employed (resolution=2 mm2, TI/TE/TR=500/4.8/2000 ms, FOV=192 mm2 ). The same conditions were applied as in the simulation. In vivo perfusion data were acquired from five healthy volunteers (3M, 26.8$$$\pm$$$2.4 years old), using a custom PICORE-PASL sequence16 (protocol details summarised in Table 1). Average perfusion signal, perfusion tSNR, and relative CBF were calculated to compare the performance of HAPY, GOIA, and trFOCI as PASL labelling pulses.
Results and discussion
Results from simulation and phantom experiments demonstrated that with the defined $$$(\Delta{B_0},B_1^+)$$$ conditions, HAPY achieved 18% energy reduction without noticeably affecting labelling profile compared to GOIA, trFOCI and HS-3 pulses. For the PICORE-PASL sequence, in the presence of saturation pulses, this translates to 6.2% SAR reduction of the protocol. The line profiles from simulation and phantom experiments (Figure 3) demonstrated that HAPY attained similar inversion accuracy in the critical passband and inversion band as GOIA and trFOCI, while all three produced higher inversion efficiency than HS-3 under the evaluated conditions.Results from in vivo scans showed no visually obvious (Figure 4a-c) or statistically significant (Figure 4d-g) difference between the perfusion results by HAPY and those by GOIA/trFOCI pulses at all three voltage levels, indicating that HAPY achieved SAR reduction without affecting the labelling efficiency. Thanks to its lower RF energy deposition, HAPY-PASL was able to achieve the highest temporal resolution within the same SAR limit, compared to GOIA and trFOCI pulses (Fig. 4h-j).
Conclusion
This study has presented a new class of asymmetric adiabatic pulses, HAPY, obtained via a systematic optimisation framework, for the application of PASL at 7T. In simulation and experiments, HAPY achieves high labelling efficiency with reduced pulse energy and thus facilitates full exploitation of the SNR benefits of ultra-high field strength for ASL.Acknowledgements
We acknowledge the facilities, and the scientific and technical assistance of the Australian National Facility, a National Collaborative Research Infrastructure (NCRIS) capability, at the Melbourne Brain Centre Imaging Unit of the University of Melbourne. The work is also supported by a research collaboration agreement with Siemens Healthineers, Australia.References
1. Alsop, D. C., Detre, J. A., Günther, M., et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine. 2015; 73: 102-116.
2. Bause, J., Ehses, P., Mirkes, C., et al. Quantitative and functional pulsed arterial spin labelling in the human brain at 9.4 T. Magnetic Resonance in Medicine. 2016; 74:1054-1063.
3. Ivanov, D., Gardumi, A., Haast, R. A. M., et al. Comparison of 3T and 7T ASL techniques for concurrent functional perfusion and BOLD stuides. NeuroImage. 2017; 156:363-376.
4. Zimmer, F., O’Brien, K., Bollmann, S., et al. Pulsed arterial spin labelling at ultra-high field with a B 1+-optimised adiabatic labelling pulse. Magnetic Resonance Materials in Physics, Biology and Medicine. 2016; 29(3): 463-473.
5. Teeuwisse, W. M., Webb, A. G., & van Osch, M. J. P. Arterial spin labeling at ultra-high field: all that glitters is not gold. International Journal of Imaging Systems and Technology. 2010; 20:62-70.
6. Frank, L. R., Wong, E. C., & Buxton, R. B. Slice profile effects in adiabatic inversion: application to multislice perfusion imaging. Magnetic Resonance in Medicine. 1997; 38(4): 558-564.
7. Silver, M. S., Joseph, R. I., & Hoult, D. I. Highly selective and π pulse generation. Journal of Magnetic Resonance. 1984; 59(2): 347-351.
8. Tannùs, A. & Garwood, M. Adiabatic pulses. NMR in Biomedicine. 1997; 10:12.
9. Edelman, R. R., Chen, Q. EPISTAR MRI: multi-slice mapping of cerebral blood flow. Magnetic Resonance in Medicine. 1998; 40:800-805.
10. Wong, E. C., Buxton, R. B., Frank, L. R. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR IN BIOMEDICINE. 1997; 10:13.
11. Yongbi, M. N., Branch, C. A., & Helpern, J. A. Perfusion imaging using FOCI RF pulses. Magnetic Resonance in Medicine. 1998; 40:938-943.
12. Duong, T. Q., Yacoub, E., Adriany, G., et al. High-resolution, spin-echo BOLD, and CBF fMRI at 4 and 7 T. Magnetic Resonance in Medicine. 2002; 48:589--593.
13. Gardener, A., Gowland, P., & Francis, S. Implementation of quantitative perfusion imaging using pulsed arterial spin labeling at ultra-high field. Magnetic Resonance in Medicine. 2009; 61:874--882.
14. Warnking, J. M. & Pike, G. B. Reducing contamination while closing the gap: BASSI RF pulses in PASL. Magnetic Resonance in Medicine. 2006; 55:865-873.
15. Hurley, A. C., Al‐Radaideh, A., & Bai, L., et al. Tailored RF pulse for magnetization inversion at ultrahigh field. Magnetic Resonance in Medicine. 2010; 63(1): 51-58.
16. Luh, W. M., Wong, E. C., Bandettini, P. A., et al. QUIPSS II with thin‐slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magnetic Resonance in Medicine. 1997; 41(6): 1246-1254.
Figures
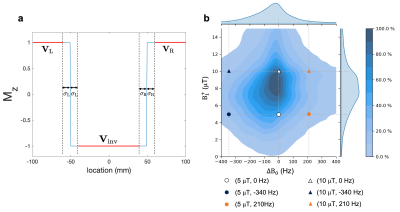
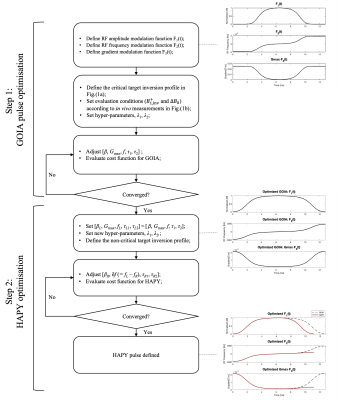
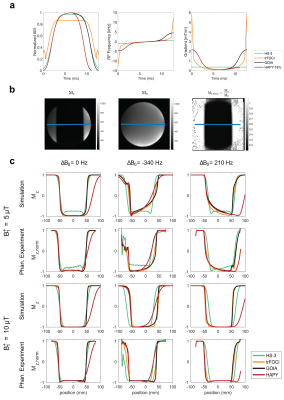
Figure 3 (a) Waveforms of the compared pulses in simulation and experiments. (b) An example of the inversion image, Mz, and the normalised inversion image, Mz,norm, obtained by Mz/M0 where M0 is the calibration image obtained with zero inversion voltage. (c) Line profiles from simulation and phantom experiments. For the phantom experiments, line profiles as marked by the blue line in (a) were created by taking the average of central three lines in the normalised inversion images.