0369
FEXI MRI detects increased blood-brain barrier water permeability in response to mild lung infection1Division of Psychology, Communication and Human Neuroscience, University of Manchester, Manchester, United Kingdom, 2Geoffrey Jefferson Brain Research Centre, University of Manchester, Manchester, United Kingdom, 3Division of Neuroscience, University of Manchester, Manchester, United Kingdom, 4UCL, London, United Kingdom, 5Danish Research Centre for Magnetic Resonance, Copenhagen, Denmark, 6Random Walk Imaging, Åkarp, Sweden, 7Evotec (UK) Ltd., Cheshire, United Kingdom, 8Bioxydyn Limited, Manchester, United Kingdom, 9Division of Informatics, University of Manchester, Manchester, United Kingdom
Synopsis
Keywords: Diffusion/other diffusion imaging techniques, Permeability
Non-disruption alterations to the blood-brain barrier (BBB) can be difficult to detect and therefore require highly sensitive tools for reliable measurement. Here, we apply a BBB filter exchange imaging (BBB-FEXI) technique to assess the rat brain in response to mild Streptococcus pneumoniae lung infection. We observe a significant 78 ± 39 % increase in BBB water permeability during infection. Higher water exchange measures were associated with higher levels of vascular inflammation, while BBB tight junction proteins remained unchanged. The expression of aquaporin-4 water channel was 38% higher in infected animals, which may drive the increase in water exchange during infection.INTRODUCTION
Blood-brain barrier (BBB) dysfunction occurs in many brain diseases, and there is increasing evidence that it is an early process in neurodegeneration [1], which may be exacerbated by peripheral infection [2]. Imaging studies have been able to detect BBB dysfunction caused by widespread inflammation from lupus [3, 4], but subtle, non-disruptive BBB alterations due to peripheral infection, can be difficult to identify, and therefore require highly sensitive tools for reliable measurement. Filter-exchange imaging (FEXI) is a promising technique that can be adapted to probe water exchange across BBB (here termed “BBB-FEXI”), as a potentially more sensitive measure of BBB alteration. BBB-FEXI has been successfully implemented in the human brain [5, 6] and attempts have been made to target brain tumours [7], but it has yet to be applied to examine subtle pathology. We have developed a preclinical BBB-FEXI approach to investigate the effects of Streptococcus pneumoniae lung infection on BBB function. We obtain measures of BBB water exchange in the rat brain before and during infection and validate against ex-vivo BBB markers: BBB tight junction proteins, aqupaporin-4 water channel protein and vascular inflammation marker, von Willibrand factor (VWF). This work demonstrates the potential of BBB-FEXI as a non-invasive tool to detect subtle BBB alterations in response to lung infection and helps to further our understanding of the impact of peripheral infection on the BBB.METHODS
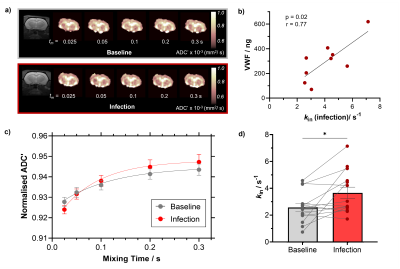
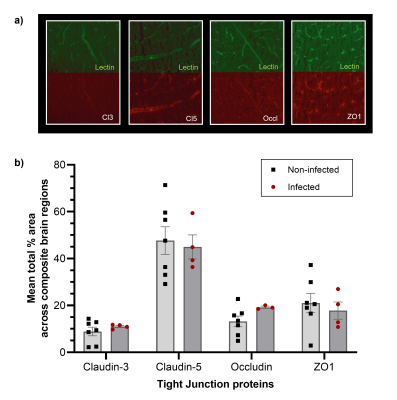
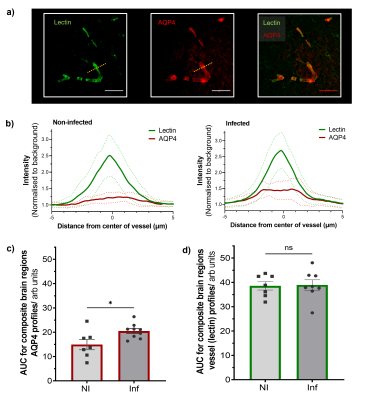
To assess the effects of peripheral infection on BBB water permeability, F334 rats (n = 14) were scanned, with a Bruker Avance III console interfaced with an Agilent 7T 16-cm bore magnet using our preclinical BBB-FEXI sequence, before infection (baseline) and again on day 8-9 of an ascending Streptococcus pneumoniae infection challenge. Imaging parameters: filter b-values (bf) = 0, 250 s/mm2; detection b = 0, 250 s/mm2; mixing times (tm) = 0.025, 0.05, 0.1, 0.2 and 0.3 s; TR = 5000 s; matrix size = 64 x 64; FOV = 32 x 32 mm2; single slice, resolution = 0.5 x 0.5 x 4.0 mm2; repetitions = 10, with a spin-echo EPI readout. Whole brain BBB water exchange (kin) estimates were obtained from ADC’(tm) measurements fitted with a novel crusher compensated exchange rate (CCXR) model.VWF levels, a peripheral marker for vascular injury and inflammation were estimated from blood plasma samples taken on the day after MRI from a subset of rats (n = 9). BBB tight junction proteins (claudin-3, claudin-5, occludin and ZO1) and aquaporin-4 (AQP4) water channel protein were assessed by immunohistochemistry staining from tissue taken on the day after MRI in the same infected rats (n = 9) and an additional cohort of non-infected F344 rats as control (n = 7). Immunohistochemistry images were taken in the frontal cortex, hippocampus and posterior cingulate and temporal cortices. The mean percentage area of the tight junction proteins was estimated and averaged across all brain regions. Vessel profiles were manually drawn on lectin images in ImageJ (Fiji), and the area-under curve (AUC) of AQP4 and lectin line profiles were taken and averaged across all brain regions. Results presented as mean ± s.e.m for all studies.
RESULTS
Infection led to a significant 78 ± 39 % increase in the BBB water exchange rate (baseline kin = 2.57 ± 0.31 s-1; infection kin = 3.67 ± 0.42 s-1; p = 0.02; Figure 1c-d). A significant positive correlation was observed between kin during infection and plasma VWF levels; p = 0.02; r = 0.77 (Figure 1b). No significant changes were measured in any of the tight junction protein markers when comparing non-infected with infected animals; claudin-3 (8.9 ± 1.8 % vs 11.0 ± 0.5 %), claudin-5 (47.7 ± 6.0 % vs 45.0 ± 5.1 %), occludin (13.2 ± 2.3 % vs 19.2 ± 0.5 %) and ZO-1 (21.0 ± 4.1 % vs 17.8 ± 7.4 %), respectively (Figure 2b). Finally, we observed a significant 38% higher AUC in AQP4 intensity profiles of infected animals (20.5 ± 1.0 arbitrary units) relative to non-infected animals (14.9 ± 2.1 arbitrary units); p = 0.02 (Figure 3c). AUC of lectin profiles remained consistent between the infected and non-infected groups (Figure 3d).DISCUSSION
BBB-FEXI detected a significant increase in kin in the rat brain in response to peripheral lung infection (Figure 1d), which demonstrates its sensitivity as a non-invasive tool to measure subtle BBB water permeability alterations such as those induced by systemic inflammation. We found the levels of plasma VWF had a strong positive correlation with kin during infection (Figure 1b), suggesting that peripheral infection could promote BBB water exchange via a non-disruptive vascular inflammatory response, since BBB tight junction protein markers remained unchanged (Figure 2b). We also found that the expression of AQP4 was 38% higher in infected animals when compared with non-infected controls (Figure 3c), which could plausibly drive the increases in water exchange observed during infection.CONCLUSION
The BBB-FEXI technique is sensitive to BBB alterations caused by lung infection and is a promising tool for detecting and monitoring early BBB dysfunction and for better understanding the impact of peripheral infection in brain diseases.Acknowledgements
This work is funded by the EPSRC: grant code EP/S031510/1. The purchase of the breeding pair and the establishment of the Fisher-344 rat strain was jointly supported by the European Union’s Seventh Framework Programme (FP7/2007-2013; grant agreement n° HEALTH-F2-2011-278850; INMiND) and Alzheimer Research UK network funds at the UoM. SL has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 804746). We would like to thank the staff at The Biological Service Facility University of Manchester for their help maintaining animal welfare and environmental enrichment during these studies.References
[1] Sweeney, M.D., A.P. Sagare, and B.V. Zlokovic, Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol, 2018. 14(3): p. 133-150.
[2] Galea, I., The blood–brain barrier in systemic infection and inflammation. Cellular & Molecular Immunology, 2021. 18(11): p. 2489-2501.
[3] Kamintsky, L., et al., Blood-brain barrier leakage in systemic lupus erythematosus is associated with gray matter loss and cognitive impairment. Ann Rheum Dis, 2020. 79(12): p. 1580-1587.
[4] Gulati, G., et al., Altered Blood-Brain Barrier Permeability in Patients With Systemic Lupus Erythematosus: A Novel Imaging Approach. Arthritis Care Res (Hoboken), 2017. 69(2): p. 299-305.
[5] Bai, R., et al., Feasibility of filter-exchange imaging (FEXI) in measuring different exchange processes in human brain. NeuroImage, 2020. 219: p. 117039.
[6] Powell, E., et al., Voxel-wise compartmental modelling of blood-brain barrier water exchange measurements using FEXI, in 31st Annual Meeting of International Society for Magnetic Resonance in Medicine. 2022: London, UK.
[7] Wang, Z., et al, Comparison of DCE-MRI and FEXI in the measurement of vascular water exchange in high-grade glioma, in 30th Annual Meeting of International Society for Magnetic Resonance in Medicine. 2021: Online.
Figures