0361
Respiratory controlled ECG-free cine construction from real-time cardiac magnetic resonance images acquired during free-breathing exercise1Clinical Physiology, Department of Clinical Sciences Lund, Lund University, Skåne University Hospital, Lund University, Lund, Sweden
Synopsis
Keywords: Data Processing, Software Tools, Exercise, real-time
This study aimed to enable ECG-free construction of respiratory controlled 3D cardiac magnetic resonance cine series from short-axis 2D real-time images through automated detection of cardiac phases. This is important for imaging during exercise, since obtaining a reliable ECG-signal can be difficult. Visually coherent cines could be constructed for midventricular slice positions through detecting cardiac phases from automated left ventricular segmentations in real-time timeframes, but not for apical and basal slice positions. Deep learning-based regression of cardiac phases directly from images was investigated to handle this problem, but apical and basal cine construction remains an open problem.
Introduction
Cardiovascular magnetic resonance (CMR) imaging during exercise can unmask early symptoms of heart disease1–3. However, breath-holding is difficult during exercise, and ECG-gating is often not reliable. Therefore, enabling retrospective construction of respiratory controlled 3D cine series from real-time 2D CMR images during free-breathing exercise without an ECG-signal could allow exercise CMR in clinical examinations.Real-time CMR allows ECG-free reconstruction, but yields limited temporal resolution. Furthermore, during free-breathing CMR, respiratory motion displaces the heart and affects ventricular filling4. To assess physiology and improve diagnostics, respiratory controlled images are needed. Current methods can identify end-diastolic (ED) and end-systolic (ES) phases5 which can yield standard measures of cardiac function. However, to obtain measures of cardiovascular hemodynamics for improved understanding of cardiac function, e.g. 4D flow6 or non-invasive pressure-volume loops7, time-resolved cines are required.
A real-time series consists of subsequently acquired timeframes in a single cardiac slice position. To construct a cine series of one cardiac cycle in one respiratory state from real-time, accurate detection and definition of respiratory and cardiac states is needed. However, analyzing hundreds of real-time timeframes for creating a single 2D cine is infeasible to do manually. Therefore, the aim of this study was to assess if retrospective construction of ECG-free respiratory controlled cines from real-time images can be enabled through automating cardiac phase detection.
Methods
DataCMR was performed on 10 healthy subjects during moderate and intensive exercise (60 % and 80 % of maximum heart rate, respectively) using a supine bicycle ergometer (Lode, Groningen, The Netherlands). Short-axis images were acquired using a real-time steady-state free precession sequence with acquired spatial resolution 1.9 x 2.8 mm, 500-800 timeframes per slice, 14-17 slice positions, and temporal resolution 34-37 ms using GRAPPA factor 3 parallel imaging and acceleration with partial Fourier factor 5/8. Previously collected breath-hold ECG-gated cine images with manual left ventricle (LV) delineations in 89 subjects were used for model development. Ethical approval was obtained. All subjects provided informed consent.
Cardiac phase detection
Respiratory signals were retrospectively estimated in each real-time series using a validated manifold learning-based method 5. A manually adaptable gating width around the desired respiratory state allowed selection of candidate timeframes for construction of the cine series. A minimum of two subsequent timeframes per partial cardiac phase were sampled to preserve temporal context.
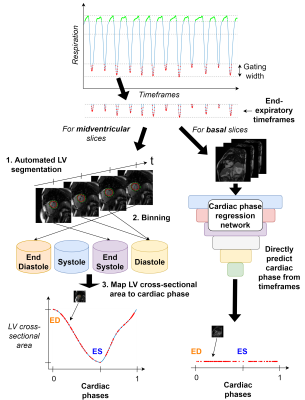
Two methods for cardiac phase detection were tested (Figure 1). First, for midventricular slices, cardiac phases were detected by: (1) segmenting the LV using deep learning-based segmentation8; (2) binning timeframes as ED, systole, ES, or diastole, using the 2D LV cross-sectional area between timeframes; (3) mapping each timeframe to a normalized cardiac phase (between 0 and 1, both being ED), using a model of 2D LV area variation during the cardiac cycle, obtained by fitting a polynomial to the average, normalized 2D LV area during systole and diastole in midventricular cine images for 89 subjects. The ratio between systolic and diastolic duration was given by manually estimating the heart rate9.
Second, due to basal LV through-plane motion, cardiac phases in basal slices were estimated using a deep regression network (Darknet1910) to predict normalized cardiac phases directly from three subsequent input timeframes. Training was performed in MATLAB R2021a using cine images with reduced spatial resolution (to synthesize the appearance of real-time images) from basal slice positions for 80 % of the 89 subjects, with remaining 18 subjects used for testing.
Sampling of cardiac phases for cine construction was performed by finding the nearest phases to a given number of phases distributed evenly across the cardiac cycle. For cine construction, motion correction was performed between timeframes by aligning any available LV center points.
Results
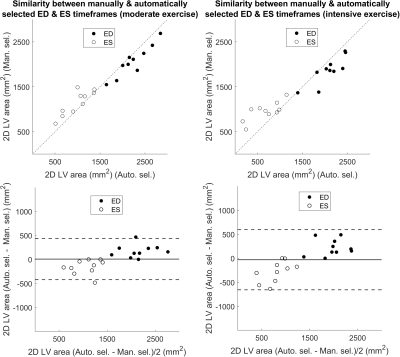
Figure 2 shows performance of the midventricular cardiac phase detection method through similarity in 2D LV area between its selected ED and ES timeframes and corresponding selections made by a physician from the same real-time series (500-800 timeframes) during end-expiration. Timeframes with similar and in several cases the very same 2D LV areas were selected.The mean absolute error between the deep regression network’s predicted cardiac phases and the ground truth phases for basal synthetic real-time images of the 18 testing subjects was 9 %. Quantitative assessments of this method for basal cine construction from exercise real-time images showed incoherence between timeframes.
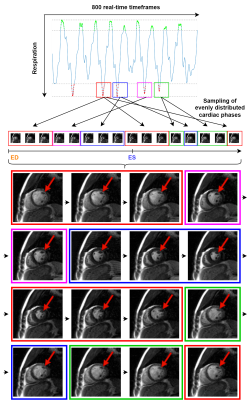
Cines constructed using the midventricular phase detection method were assessed qualitatively (Figure 3). Although pieced together from partial cardiac cycles from different temporal positions, the positions of the papillary muscles were coherent. Video 1 shows a midventricular cine constructed during moderate exercise, and Video 2 shows the same slice position during intensive exercise, indicating that increased exercise intensity somewhat impairs coherence.
Discussion
The precision (in ED and ES selection) of the midventricular cardiac phase detection method and the temporal coherence of anatomical structures in constructed examples showed feasibility of constructing respiratory controlled cine series from midventricular real-time exercise CMR images. Deep cardiac phase regression needs further improvements to enable robust cine construction for apical and basal slices.Conclusion
Coherent respiratory controlled cine series can be constructed from midventricular real-time 2D CMR series during free-breathing exercise without cardiac gating through automated cardiac phase detection. However, apical and basal slices remain an open problem.Acknowledgements
No acknowledgement found.References
1. Jaijee S, Quinlan M, Tokarczuk P, et al. Exercise cardiac MRI unmasks right ventricular dysfunction in acute hypoxia and chronic pulmonary arterial hypertension. Am J Physiol-Heart Circ Physiol. 2018;315(4):H950-H957.
2. Craven TP, Tsao CW, La Gerche A, Simonetti OP, Greenwood JP. Exercise cardiovascular magnetic resonance: development, current utility and future applications. J Cardiovasc Magn Reson. 2020;22(1):65.
3. Backhaus SJ, Lange T, George EF, et al. Exercise Stress Real-Time Cardiac Magnetic Resonance Imaging for Noninvasive Characterization of Heart Failure With Preserved Ejection Fraction: The HFpEF-Stress Trial. Circulation. 2021;143(15):1484-1498.
4. Claessen G, Claus P, Delcroix M, Bogaert J, Gerche AL, Heidbuchel H. Interaction between respiration and right versus left ventricular volumes at rest and during exercise: a real-time cardiac magnetic resonance study. Am J Physiol-Heart Circ Physiol. 2014;306(6):H816-H824.
5. Edlund J, Haris K, Ostenfeld E, et al. Validation and quantification of left ventricular function during exercise and free breathing from real-time cardiac magnetic resonance images. Sci Rep. 2022;12(1):5611.
6. Carlsson M, Heiberg E, Toger J, Arheden H. Quantification of left and right ventricular kinetic energy using four-dimensional intracardiac magnetic resonance imaging flow measurements. Am J Physiol-Heart Circ Physiol. 2012;302(4):H893-H900.
7. Seemann F, Arvidsson P, Nordlund D, et al. Noninvasive Quantification of Pressure-Volume Loops From Brachial Pressure and Cardiovascular Magnetic Resonance. Circ Cardiovasc Imaging. 2019;12(1).
8. Berggren K, Hedstrom E, Ehrenborg KS, et al. Multiple Convolutional Neural Networks for Robust Myocardial Segmentation. In proceedings of SSBA 2020.
9. Chung CS, Karamanoglu M, Kovács SJ. Duration of diastole and its phases as a function of heart rate during supine bicycle exercise. Am J Physiol-Heart Circ Physiol. 2004;287(5):H2003-H2008.
10. Redmon J, Farhadi A. YOLO9000: better, faster, stronger. In: Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition. 2017;7263-7271.
Figures