0341
High on Sparsity: Inter-Bin Compensation of Cardiac Motion for Improved Assessment of Left Ventricular Function Using 5D whole-heart MRI1Department of Diagnostic and Interventional Radiology, Lausanne University Hospital, Lausanne, Switzerland, 2Center for Biomedical Imaging (CIBM), Lausanne, Switzerland, 3Woman-Mother-Child Department, Lausanne University Hospital, Lausanne, Switzerland
Synopsis
Keywords: Heart, Image Reconstruction
The reconstruction of 5D cardiac and respiratory motion-resolved images with the free-running framework (FRF) requires careful optimization of the regularization weights to avoid compression of physiological motion, which in turn may lead to underestimated left ventricular ejection fraction (LVEF). This study presents a novel cardiac and respiratory motion-resolved reconstruction with inter-bin compensation of cardiac motion. We demonstrate that the proposed framework significantly improves image quality while preserving accurate LVEF when compared to the original 5D framework. This work highlights important considerations when reconstructing cardiac resolved images without compensation and provides a robust approach for the assessment of LVEF.INTRODUCTION
Left ventricular ejection fraction (LVEF) is a central parameter for risk stratification of patients with heart failure1 and cardiac magnetic resonance imaging (CMR) plays an important role in its assessment. Recently, the free-running framework2 (FRF) was proposed to potentially simplify the workflow for LVEF assessment by acquiring 3D whole-heart data continuously without the need for ECG placement or respiratory navigators, then retrospectively reconstructing fully self-gated cardiac and respiratory motion-resolved (5D) images. To reconstruct the highly undersampled images, a compressed sensing algorithm assumes that the difference between two adjacent frames is sparse and requires careful optimization of the regularization weights to find a compromise between residual aliasing and compression of the underlying physiological motion. While image quality generally improves with higher regularization weights, it results in higher compression of physiological motion, which may lead to an underestimation of LVEF.3 We hypothesize that the incorporation of deformation fields, which describe cardiac motion occurring between adjacent cardiac cine frames, into the regularization term of the reconstruction problem4 will better preserve cardiac motion, improve image quality, and LVEF measurements. This work presents an inter-bin cardiac motion-compensated technique for 5D CMR. We optimize this approach using a comprehensive numerical simulation, validate its feasibility in volunteers, and demonstrate its ability to improve image quality compared to the uncompensated approach, while preserving accurate LVEF.METHODS
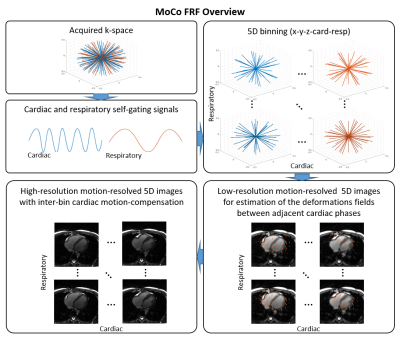
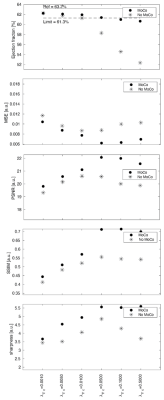
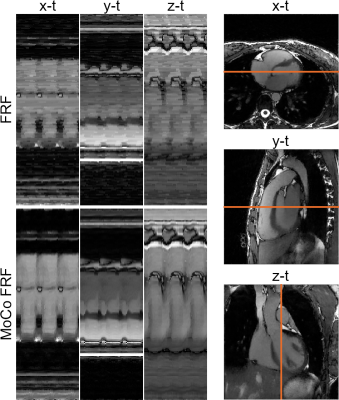
A schematic overview of the proposed framework for inter-bin cardiac motion compensation is illustrated in Fig.1. The framework was first validated in a numerical simulation based on the MRXCAT5 and XCMR6 approaches. The simulated free-running data were generated to match the in vivo data (described below) and included variable nonrigid cardiac and respiratory motion, and a LVEF of 63.2%. To demonstrate the feasibility of our proposed framework in vivo, 4 volunteers were scanned with a prototype free-running non-ECG-triggered 3D golden-angle radial bSSFP sequence on a 1.5T MRI scanner (MAGNETOM Aera, Siemens Healthcare) with the following parameters: matrix (192)3, resolution (1.1mm)3, TE/TR=1.91/3.9ms, RF excitation angle =67°, 126478 total readouts continuously acquired over 8:18 min. Cardiac and respiratory self-gating signals were obtained as described previously2 and used to sort the data into 4 respiratory states and cardiac phases of 50ms. The resultant 5D (x-y-z-cardiac-respiratory dimensions) datasets were reconstructed using both the proposed inter-bin cardiac motion-compensated approach (MoCo-FRF, Fig.1) and the previously published FRF approach for comparison.2 In brief, the proposed approach consists of a two-step procedure. First, we estimate the deformation field between consecutive cardiac phases and second, we incorporate the deformation field into the reconstruction to align inter-bin cardiac motion, further sparsifying the residual between cardiac frames (Fig.1). The numerical simulations and in vivo data were reconstructed with and without motion compensation. For the numerical simulation, the cardiac regularization weights were varied between 0.001 and 0.5. The compression of cardiac motion was quantified by measuring the LVEF relative to the ground truth image, while the image quality was quantitatively assessed by measuring the following metrics: mean squared error (MSE), structural similarity index measure (SSIM), peak signal-to-noise ratio (PSNR), and blood-myocardium interface sharpness. The regularization weights that yielded the highest image quality with an LVEF error below 3% when compared to ground truth were selected for the in vivo reconstructions. For the in vivo images, LVEF was measured using a commercially available software (cvi42, Circle Cardiovascular Imaging Inc., Calgary) and blindly compared with the LVEF values of the reconstruction without motion compensation.RESULTS
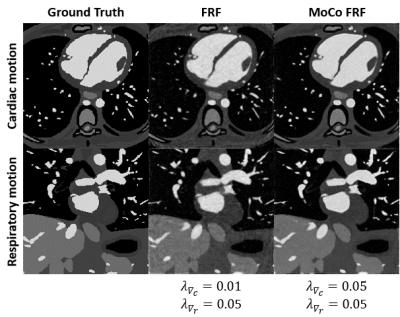
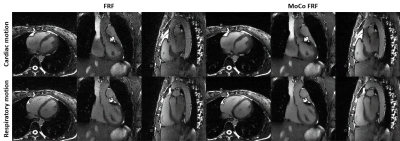
Increasing the regularization weights without motion compensation generally resulted in a decrease in LVEF and an improvement in image quality, but only until a certain point beyond which image quality degraded again (Fig.2). In contrast, the reconstruction with inter-bin cardiac motion-compensation better preserved LVEF and resulted in higher image quality overall. The regularization weights that yielded the highest image quality with an LVEF error below 3% were $$$\lambda_{\nabla_c}$$$=0.05 and $$$\lambda_{\nabla_c}$$$=0.01 for the reconstruction with and without motion-compensation, respectively. The animated depiction of the 5D reconstructions in Fig.3 demonstrates that image quality is significantly higher using motion compensation. Reconstructions with and without motion-compensation of the in-vivo FRF datasets using these optimized regularization weights resulted in LVEF of 51.5% and 50.2% (p=0.6), respectively. Fig. 4 provides an animated depiction of 5D in vivo images in a volunteer using the optimized regularization parameters and shows a clear improvement in image quality with motion compensation. Finally, Fig. 5 shows the corresponding signal evolution along the cardiac phases, which depicts significantly higher image quality and details for the motion-compensated approach.DISCUSSION AND CONCLUSIONS
This study presents a novel framework for 5D whole-heart MRI with inter-bin compensation of cardiac motion for improved assessment of LVEF. Using a numerical phantom with well-controlled boundary conditions, quantitative assessment of LVEF and multiple image quality metrics demonstrated significant improvements when using the proposed framework and it helped optimize parameter range for in-vivo reconstructions. In vivo findings corroborated the results from numerical simulations. Incorporating inter-bin cardiac motion compensation enables significant improvement of image quality with higher regularization weight along the cardiac dimension while preserving accurate LVEF assessment. Overall, this work not only highlights important considerations when reconstructing cardiac resolved images without compensation, but also provides a robust and accurate solution for LVEF assessment.Acknowledgements
No acknowledgement found.References
1. Curtis, J. P. et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol 42, 736–42 (2003).
2. Di Sopra, L., Piccini, D., Coppo, S., Stuber, M. & Yerly, J. An automated approach to fully self-gated free-running cardiac and respiratory motion-resolved 5D whole-heart MRI. Magn. Reson. Med. 82, 2118–2132 (2019).
3. Yerly, J. et al. Numerical optimization of 5D cardiac and respiratory motion-resolved CMR imaging for the assessment of left ventricular function. in Proc. Intl. Soc. Mag. Reson. Med. 1650 (2022).
4. Asif, M. S., Hamilton, L., Brummer, M. & Romberg, J. Motion-adaptive spatio-temporal regularization for accelerated dynamic MRI. Magn. Reson. Med. 70, 800–812 (2013).
5. Wissmann, L., Santelli, C., Segars, W. P. & Kozerke, S. MRXCAT: Realistic numerical phantoms for cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 16, 63 (2014).
6. Roy, C. W. et al. Motion compensated whole-heart coronary cardiovascular magnetic resonance angiography using focused navigation (fNAV). J. Cardiovasc. Magn. Reson. 23, 33 (2021).
Figures