0326
Hyperpolarized 13C Pyruvate + Urea Dual Agent MRI Detects Hypoxia-Driven Metabolism Perfusion Mismatch in Patient w/ Aggressive Prostate Cancer1Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, San Francisco, CA, United States
Synopsis
Keywords: Prostate, Cancer
The first-in-human hyperpolarized [1-13C]pyruvate+[13C,15N2]urea dual-agent MRI demonstrated the safety and feasibility of simultaneous characterization of prostate cancer metabolism and blood flow as a two-minute addition to the standard 1H-multiparametric MRI. Metabolism-perfusion mismatch (i.e. elevated pyruvate-lactate conversion and decreased urea perfusion) in subregions of the high-grade prostate tumor was in agreement with the histopathological and immunochemical markers that reflected lethal phenotypes and their associated hypoxic tumor microenvironment. Correlative analysis of urea and Gadolinium-derived pharmacokinetic parameters found low correlation between the two, which indicates that HP 13C urea’s unique contrast mechanism offers independent information inaccessible through conventional 1H DCE-MRI.Purpose
Prostate cancer impacts 1 out of 6 US men1, but many with low-risk disease suffer from excess morbidity associated with overdiagnosis and overtreatment, and there is an unmet clinical need to distinguish lethal from indolent cancer forms. Preclinical hyperpolarized (HP) 13C pyruvate+urea MRI revealed that significantly elevated pyruvate-lactate metabolism and decreased urea perfusion, reflective of hypoxia response signaling, are hallmarks of aggressive (high-grade) prostate cancer phenotypes in mice2,3. This first-in-human research explored the safety and feasibility of simultaneous metabolism and perfusion/permeability characterization using HP [1-13C]pyruvate+[13C,15N2]urea dual-agent MRI in a patient with histologically-confirmed high-grade prostate cancer. Correlative analysis was performed between HP 13C-1H pharmacokinetic parameters and histopathological/immunochemical markers.Methods
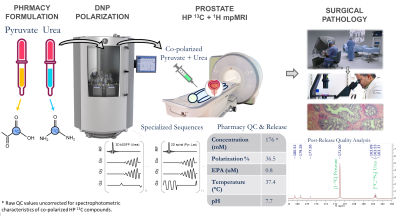
Patient Characteristics: A patient with biopsy-confirmed high-grade prostate cancer (Gleason 4+3, PSA = 7.7) was enrolled via an IRB-approved protocol (NCT02526368). Key eligibility criteria include biopsy-confirmed prostate cancer, planned radical prostatectomy, and ECOG status of 0 or 1.Hyperpolarized-13C Exam: GMP [1-13C]pyruvic acid and [13C,15N2]urea were co-polarized in a 5T SpinLab (GE Healthcare, Chicago IL) polarizer for 3 hours4. The dissolution yielded 125/25 mM pyruvate/urea solution with 36.5% polarization and 0.8μM residual trityl radical. Imaging was conducted on a clinical 3T scanner (MR750, GE Healthcare) using a clamshell 13C transmitter and a dual-element 1H-13C endorectal receiver5. Pharmaceutical release followed QC verification of IND-approved safety criteria4. Post-release quality analysis used a high-field spectrometer (Varian INOVA 500).
Imaging Sequence: Time-resolved pyruvate-lactate imaging used a 2D multislice single-shot spiral sequence (TR =80ms) with 7x7x11.6mm spatial resolution6, whereas urea used a 3D stack-of-spiral balanced-SSFP sequence (TR = 12.3ms, α = 50°) at 9x9x11.6mm resolution7,8. Scan time window was 52s with 2.6s temporal resolution.
Data Processing and Analysis: Quantitative 1H-DCE parameters (kTRANS, ve) were calculated with 3D Slicer’s PkModeling toolbox9, using a femoral artery ROI in the prostate plane as arterial input. Semi-quantitative DCE parameters (peak height, slope, washout) were derived using an in-house pipeline10, and those of urea were calculated in MATLAB. Pairwise Pearson correlation used voxels within the prostate ROI, with DCE parametric maps downsampled to 13C resolution. Pyruvate-to-lactate conversion rate kPL was calculated using an inputless two-site model11. Spatial distributions of all 13C tracers were corrected for receiver coil sensitivity profile.
Results and Discussions
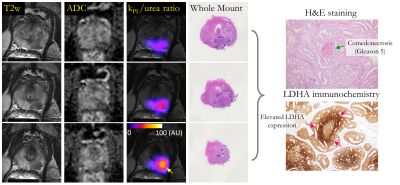
The first-in-human co-hyperpolarized [13C,15N2] urea and [1-13C]pyruvate as a two-minute addition to 1H multiparametric MRI of prostate cancer was safe and feasible without adverse events. The novel workflow (Figure 1) features pharmaceutical compounding of 13C-labelled pyruvate+urea4, specialized MR coils5, pulse sequences6-8, quantification of novel biomarkers, along with QC and release criteria for co-polarized HP agents4.Surgical pathology from radical prostatectomy identified Gleason 4+5 tumor in the left posterior mid-apex peripheral zone (PZ), confirming the high-risk diagnosis (UCSF CAPRA score 5, Decipher 0.84)12. The Gleason pattern 5 consisted of solid sheets of cells and comedonecrosis.
Composite biomarker kPL/ureaAUC ratio (kUR) looks at increased pyruvate metabolism coupled with decreased urea perfusion. This metabolism-perfusion mismatch is a signature of the hypoxia-driven lethal, treatment-resistant prostate tumor phenotypes2,3,13,14, where overdrive of hypoxia-induced factor Hif1α signaling pathway leads to high glycolysis (Warburg effect) and hyperproliferation15,16. Heterogeneous kUR (Figure 2) was observed intratumorally with subregions (yellow arrow) as high as 10x the normal-appearing PZ. Indeed, immunohistochemical analysis identified presence of comedonecrosis (green arrow, Gleason 5) with significantly stronger lactate dehydrogenase (LDHA; a direct downstream target of Hif1α upregulated in hypoxic tumor microenvironment17) immunochemical staining surrounding a central area of necrosis (red arrow) and within intraductal carcinoma component.
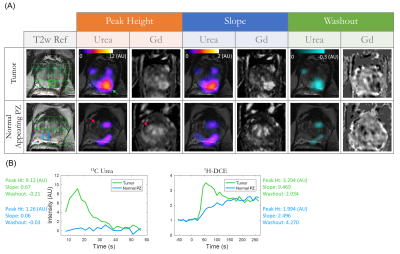
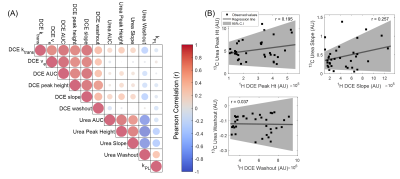
The semi-quantitative parametric maps (Figure 3) showed focal urea hyperintensity at the histologically-confirmed left PZ tumor (green arrow), whereas the central gland BPH nodules had low urea (red arrow), as opposed to 1H-DCE which had moderate enhancement. Time-resolved urea vs Gd-enhanced T1-weighted signal in tumor and normal-appearing PZ voxels showed that urea distributed much more rapidly throughout the tumor than Gadolinium, but also decreased rapidly - possibly through combined washout and T1-decay mechanisms. General correlation and pairwise regression diagrams (Figure 4) between urea and DCE pharmacokinetic biomarkers gave weak correlation (Pearson r2mean = 0.04, range:0.001-0.17), indicating that HP 13C urea provided unique, independent perfusion information not accessible through standard 1H contrast-enhanced MRI.
A possible explanation for HP 13C urea’s unique contrast mechanism is that urea is a highly polar, diffusive small-molecule agent that is particularly sensitive to permeability differences in the microvasculature18,19. Urea extravasates more rapidly from the highly permeable, leaky angiogenic tumor neovasculature into the interstitial space, than it does from the highly vascularized but less permeable benign central gland nodules, analogous to von Morze’s findings of much higher urea signal levels in the highly permeable mouse tumor than the poorly permeable brain18. These preliminary findings warrant future investigations of composite metabolism-perfusion biomarkers in a larger cohort of patients for early detection of aggressive prostate tumors, and illustrated the need to develop more sophisticated quantitative modeling of urea perfusion/permeability to fully exploit the wealth of information it has to offer.
Conclusions
This first-in-human co-hyperpolarized [13C,15N2] urea and [1-13C]pyruvate + 1H mpMRI of prostate cancer demonstrated safety and feasibility of simultaneous characterization of perfusion and metabolism in human malignancies using hyperpolarized agents. Metabolism-perfusion mismatch detected hypoxia-driven aggressive cancer subtypes, consistent with pathological/immunochemical findings. Carbon-13 urea biomarkers provided unique perfusion information inaccessible through conventional 1H DCE-MRI.Acknowledgements
This work was supported by grants from the NIH (U01EB026412, R01CA214554, U01CA232320, P41EB013598). We would like to thank Priscilla Chan, Heather Daniel, Evelyn Escobar, Mary Frost, Jasmine Hu, Dr. Yaewon Kim, Dr. Philip Lee, Kimberly Okamoto, Dr. Andrew Riselli, and Dr. James Slater for their help with this research.References
[1] Cancer Facts and Figures 2022. American Cancer Society, Atlanta, GA (2022).
[2] Chen HY et al., Assessing prostate cancer aggressiveness with hyperpolarized dual‐agent 3D dynamic imaging of metabolism and perfusion. Cancer Res. 2017; 77(12), 3207‐3216
[3] Bok RA et al., The Role of Lactate Metabolism in Prostate Cancer Progression and Metastases Revealed by Dual-Agent Hyperpolarized 13C MRSI. Cancers 2019; 11(2), 257
[4] Qin H et al., Clinical translation of hyperpolarized 13C pyruvate and urea MRI for simultaneous metabolic and perfusion imaging. Magn Reson Med. 2022; 87(1), 138-149
[5] Nelson SJ et al., Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Sci Transl Med 2013; 5(198), 198ra108
[6] Tang S et al., A regional bolus tracking and real‐time B1 calibration method for hyperpolarized 13C MRI. Magn Reson Med 2019; 81(2), 839-851
[7] Tang S et al., A metabolite‐specific 3D stack‐of‐spiral bSSFP sequence for improved lactate imaging in hyperpolarized [1‐13C]pyruvate studies on a 3T clinical scanner. Magn Reson Med 2019; 84(3), 1113-1125
[8] Liu X et al., Development of specialized magnetic resonance acquisition techniques for human hyperpolarized [13C, 15N2] urea+[1‐13C] pyruvate simultaneous perfusion and metabolic imaging. Magn Reson Med 2022; doi: 10.1002/mrm.28965
[9] Miller J et al., PkModeling: Slicer Extension providing pharmacokinetic modeling. C++. 2018. https://github.com/millerjv/PkModeling
[10] Noworolski, SM et al., Dynamic contrast-enhanced MRI and MR diffusion imaging to distinguish between glandular and stromal prostatic tissues. Magn. Reson. Imaging 2008; 26(8), 1071-1080
[11] Larson PEZ et al., Investigation of analysis methods for hyperpolarized 13C‐pyruvate metabolic MRI in prostate cancer patients. NMR Biomed 2018; 31(11), e3997.
[12] Cooperberg MR et al., The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J. Urol. 2005; 173(6), 1938-1942
[13] Mankoff DA et al., Blood flow-metabolism mismatch: good for the tumor, bad for the patient. Clin Cancer Res. 2009; 15(17), 5294-5296
[14] Miles KA et al., Warburg revisited: imaging tumour blood flow and metabolism. Cancer Imaging 2008; 8(1), 81
[15] Tran MGB et al., Independence of HIF1a and androgen signaling pathways in prostate cancer. BMC cancer 2020; 20(1), 1-12
[16] Majumder PK et al., mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nature medicine 2004; 10(6), 594-601
[17] Semenza, GL et al., Tumor metabolism: cancer cells give and take lactate. The Journal of clinical investigation 2008; 118(12), 3835-3837
[18] von Morze C et al., Simultaneous multiagent hyperpolarized 13C perfusion imaging. Magn Reson Med. 2022; 72(6), 1599-1609
[19] Patrick PS et al., Detection of transgene expression using hyperpolarized 13C urea and diffusion‐weighted magnetic resonance spectroscopy. Magn Reson Med. 2022; 73(4), 1401-1406
Figures