0320
Human Prostate MRI at ultra-high-performance gradient of 200 mT/m and 500 T/m/s1GE Research, Niskayuna, NY, United States, 2Brigham and Women's Hospital, Boston, MA, United States, 3Memorial Sloan Kettering Cancer Center, New York, NY, United States, 4Lund University, Lund, Sweden
Synopsis
Keywords: Prostate, Diffusion/other diffusion imaging techniques, Tumor, Microstructure
Diffusion MRI has been shown to yield imaging biomarkers for prostate cancer identification and grading. However, the image quality remains poor due to the limited gradient performance of clinical whole-body MRI systems. We assessed the technical feasibility of human prostate MRI in a 42-cm inner-diameter gradient coil operating at maximum gradient amplitude of 200 mT/m and slew rate of 500 T/m/s. No sensation of peripheral nerve stimulation was reported. Significantly reduced image distortion and higher image signal-to-noise ratios were achieved in diffusion MRI due to shorter echo times and shorter echo spacings, compared to diffusion MRI at whole-body MRI systems.INTRODUCTION
Prostate cancer is the second most commonly occurring cancer in men1. Diffusion weighted imaging (DWI) and the derived apparent diffusion coefficient (ADC) plays an essential role for the detection of clinically significant cancer. However, standard clinical diffusion MRI with echo planar acquisition suffers from image distortion due to the long echo spacing, especially in posterior peripheral zone prostate gland – where most of the cancers are located. Furthermore, diffusion MRI also suffers from relatively low signal-to-noise ratio (SNR) due to the prolonged echo time (TE) caused by the long diffusion encoding duration. The compromised image quality and clinical performance of diffusion MRI are directly affected by the gradient performance of clinical whole-body MRI systems, where the maximum gradient amplitude is 33-100 mT/m and slew rate is 150-200 T/m/s. Ultra-high-performance gradients with simultaneously high gradient amplitude and slew rate could be very advantageous. The purpose of this study was to evaluate the technical feasibility of in vivo prostate MRI in an ultra-high-performance human 3.0T system that can simultaneously achieve high gradient amplitude and slew rate.METHODS
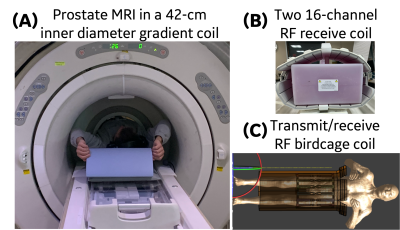
MRI experiments were performed at a 3.0T MRI system (MR750, GE Healthcare, USA) with a 42-cm inner diameter MAGNUS gradient coil2, which achieves maximum gradients of 200 mT/m and 500 T/m/s for each gradient axis. Figure 1 shows the prostate imaging setup. The dedicated transmit/receive birdcage radiofrequency (RF) coil reduces the inner diameter to 37 cm. Two 16-channel phased-array receive RF coils (GEM Flex, NeoCoil, LLC, USA) were placed anterior and posterior around the pelvis.As a dedicated transmit/receive RF coil was used, we evaluated specific absorption rate (SAR) safety by performing electromagnetic simulations (Sim4Life, Zurich MedTech, Switzerland) using a male body model3 at prostate landmark. Electromagnetic transmit magnetic field (B1+) and specific absorption rate (SAR) distributions from the simulations were evaluated for the prostate exposed mass region.
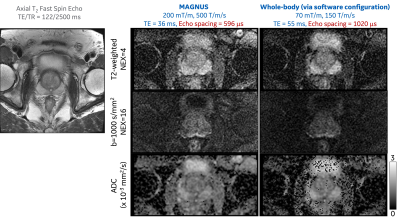
Four participants (waist circumference=81-91 cm, age=45-65 years) were recruited and scanned under an IRB-approved protocol. Each participant was scanned with clinical PI-RADS V2.1 compliant axial T2-weighted anatomical imaging and DWI (TE=55 ms at b-value=1000 s/mm2) at maximum gradients of 70 mT/m and 150 T/m/s (Table 1). In addition, a second DWI (TE=36 ms) was acquired at maximum gradients of 200 mT/m and 500 T/m/s (Table 1), and the peripheral nerve stimulation (PNS) threshold of the MAGNUS coil2, 4, 5. Each participant was provided a squeeze-bulb alarm and was instructed to use it when feeling any PNS sensation during the scan.
ADC was estimated separately from the two DWI acquisitions. Gradient nonlinearity correction was applied for diffusion encoding following the established approach6.
RESULTS
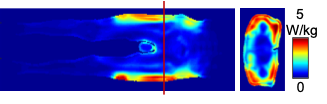
Figure 2 shows the simulated map of the local SAR averaged over a 2 mm axial slice thickness over 10 g of tissue. The peak local SAR, normalized to 2 μT, was identified in the glute muscle. At the prostate landmark, the average and peak local SAR were 1.17 and 6.29 W/kg respectively. The peak local SAR scaled to the 3.2 W/kg IEC SAR limit is 17.2 W/kg, which is less than the IEC limit7 of 20 W/kg.The image acquisitions of all the four participants were successfully completed. No PNS sensation was reported during DWI acquisitions.
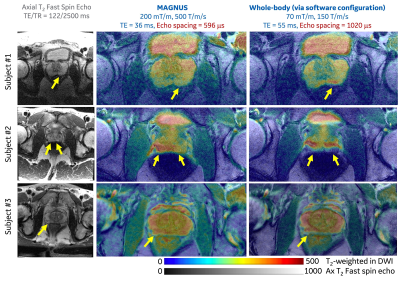
Image distortion of DWI at ultra-high-performance gradient, especially in regions near the prostate-rectum boundaries, was significantly reduced in three participants (Figure 3). Higher SNR of T2-weighted image in the DWI acquisition was observed at a shorter echo time achieved by using the ultra-high-performance gradient, as indicated by the preserved signals of muscles surrounding the prostate. The image contrast between the peripheral zone and the transition zone became lower, due to the shortening of TE which results in reduced T2 effect. Furthermore, the estimation of ADC was more robust due to the higher SNR.
DISCUSSION AND CONCLUSION
Our study has demonstrated, for the first time, the feasibility of performing prostate MRI at simultaneously high amplitude and slew rate (200 mT/m, 500 T/m/s) in the 42-cm inner diameter gradient coil. In comparison, the Connectome study8 only achieved high amplitude (300 mT/m, 200 T/m/s). Initial electromagnetic simulations indicated that patient scanning at a prostate landmark on MAGNUS gradient coil did not exceed the IEC SAR safety limits. Four healthy participants with a waist circumference<92 cm, which represents ~15% of men over 40 years old in the United States9, were all able to fit in the scanner.None of the four participants reported PNS sensations during echo planar imaging that utilized a maximum gradient slew rate of 500 T/m/s, indicating that a ~5X higher PNS threshold can be achieved for prostate imaging in the MAGNUS imaging platform. The high slew rate results in 58% shorter echo spacing, compared to that in clinical whole-body MRI systems, resulting in significantly reduce image distortion.
The high gradient amplitude results in a shorter TE of 36 ms in DWI at b-value of 1000 s/mm2, while TE is 55 ms in clinical whole-body MRI systems. The shortened TE will result in 15-25% higher SNR assuming T2 of 85-140 ms in prostate gland10.
In conclusion, human in vivo prostate MRI at 200 mT/m, 500 T/m/s, and a high PNS threshold is feasible in the ultra-high-performance MAGNUS MRI system.
Acknowledgements
The authors thank Dr. Eugene Milshteyn and Dr. Arnaud Guidon for their suggestions on the study design and discussion on the results, as well as Dr. Liang Xuan for the help on the configuration of radiofrequency receive coil.References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer Journal for Clinicians. 2022;72(1):7-33. doi: https://doi.org/10.3322/caac.21708.
2. Foo TK, Tan ET, Vermilyea ME, Hua Y, Fiveland EW, Piel JE, Park K, Ricci J, Thompson PS, Graziani D. Highly efficient head‐only magnetic field insert gradient coil for achieving simultaneous high gradient amplitude and slew rate at 3.0 T (MAGNUS) for brain microstructure imaging. Magnetic resonance in medicine. 2020;83(6):2356-69.
3. Gosselin M-C, Neufeld E, Moser H, Huber E, Farcito S, Gerber L, Jedensjö M, Hilber I, Gennaro FD, Lloyd B, Cherubini E, Szczerba D, Kainz W, Kuster N. Development of a new generation of high-resolution anatomical models for medical device evaluation: the Virtual Population 3.0. Physics in Medicine and Biology. 2014;59(18):5287-303. doi: 10.1088/0031-9155/59/18/5287.
4. Lee S-K, Mathieu J-B, Graziani D, Piel J, Budesheim E, Fiveland E, Hardy CJ, Tan ET, Amm B, Foo TK-F, Bernstein MA, Huston III J, Shu Y, Schenck JF. Peripheral nerve stimulation characteristics of an asymmetric head-only gradient coil compatible with a high-channel-count receiver array. Magnetic Resonance in Medicine. 2016;76(6):1939-50. doi: https://doi.org/10.1002/mrm.26044.
5. Tan ET, Shih RY, Mitra J, Sprenger T, Hua Y, Bhushan C, Bernstein MA, McNab JA, DeMarco JK, Ho VB. Oscillating diffusion‐encoding with a high gradient‐amplitude and high slew‐rate head‐only gradient for human brain imaging. Magnetic resonance in medicine. 2020;84(2):950-65.
6. Tan ET, Marinelli L, Slavens ZW, King KF, Hardy CJ. Improved correction for gradient nonlinearity effects in diffusion‐weighted imaging. Journal of Magnetic Resonance Imaging. 2013;38(2):448-53.
7. Commission IE. Medical electrical equipment-Part 2-33: Particular requirements for the basic safety and essential performance of magnetic resonance equipment for medical diagnosis. IEC 60601-2-33 Ed 30. 2010.
8. Molendowska M, Fasano F, Rudrapatna U, Kimmlingen R, Jones DK, Kusmia S, Tax CMW, Evans CJ. Physiological effects of human body imaging with 300 mT/m gradients. Magnetic Resonance in Medicine. 2022;87(5):2512-20. doi: https://doi.org/10.1002/mrm.29118.
9. Fryar CD, Carroll MD, Gu Q, Afful J, Ogden CL. Anthropometric reference data for children and adults: United States, 2015-20182021.
10. Hoang Dinh A, Souchon R, Melodelima C, Bratan F, Mège-Lechevallier F, Colombel M, Rouvière O. Characterization of prostate cancer using T2 mapping at 3T: A multi-scanner study. Diagnostic and Interventional Imaging. 2015;96(4):365-72. doi:
Figures