0318
Decomposition of clinical ADC into intracellular and extracellular-extravascular contributions in prostate cancer using histology1Radiomics Group, Vall d'Hebron Institute of Oncology, Barcelona, Spain, 2Department of Computer Science, Centre for Medical Image Computing, University College London, London, United Kingdom, 3Department of Pathology, University College London Hospitals NHS Foundation Trust, London, United Kingdom, 4Centre for Medical Imaging, University College London, London, United Kingdom
Synopsis
Keywords: Prostate, Cancer, Histology
Diffusion MRI has shown promising results for characterizing prostate cancer. However, diagnostic clinical apparent diffusion coefficient (ADC) has limited specificity and interpretability. Towards addressing these limitations, we aim to unravel clinical ADC into microstructural components using histology.
Histology from two prostatectomies with prostate cancer (Gleason 3+4, 4+3) were analysed in benign, inflammation and cancer regions. Cell and tissue properties were used to decouple clinical ADC into intracellular, extracellular-extravascular ADCs.
Results revealed significantly low intracellular ADC in cancer. Low ADC was also found for inflammation, which could explain ADC’s low specificity, demonstrating the added value of histology data for clinical ADC.
Introduction
Diffusion Magnetic Resonance Imaging (dMRI) exploits the Brownian diffusive motion of water molecules in tissues to sensitise MRI signals to the salient characteristics of cellular microstructure1,2. One of the most common dMRI metrics obtained in clinical settings is the Apparent Diffusion Coefficient (ADC). ADC is a surrogate index that is highly sensitive to alterations in local cellular microenvironments. However, it is also highly unspecific, as it is influenced by several different factors, implying that changes in ADC can be difficult to interpret. An example of this issue is seen in prostate imaging, where low ADC values can be found both in benign and malignant tissue regions3,4. In this work, we aim to pinpoint specific contributions to clinical ADC measurements in benign and malignant prostate lesions, to inform the interpretation of ADC changes in clinical settings. To this end, we use information from histological images obtained through prostatectomies, and use it to decompose ADC into its intracellular (IC) and extracellular-extravascular space (EES) contributions in co-localised clinical ADC maps.Methods
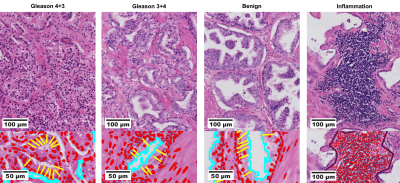
Prostate cancer digitalized histological assays (3 μm slices from 5 mm sections) with hematoxylin and eosin staining from two prostatectomies5 (Gleason 3+4 and 4+3) were collected (Figure 1, top). In addition, diffusion MRI scans acquired before prostatectomy on a 3T Philips Achieva scanner were also available. The diffusion MRI protocol, originally developed for VERDICT2,6 imaging, included five non-zero b-values [90; 3000 s/mm2]. In this study, we focus on clinical ADC mapping performed using VERDICT MRI images acquired at b = [0; 1500] s/mm2 with TE = 90 ms, TR = 2482 ms, δ = 23.9 ms, Δ = 43.8 ms. Regions of interest (ROIs) of benign, malignant and inflammation areas (Figure 1, bottom) were extracted from histology as marked by an experienced histopathologist. Individual cell nuclei, cellularity (cells over mm2 area), cell longest diameters, IC and EES fractions were measured with the software QuPath7.Characteristic volume-weighted cell size8 $$$\mathrm{L}$$$ was inferred from individual cell diameters $$$\mathrm{l}$$$ as:
$$\mathrm{L=\left(\frac{\lt l^7\gt }{\lt l^3\gt }\right)^\frac 14}$$
This was used to estimate intracellular ADCIC for the given clinical-like acquisition protocol (δ = 23.9 ms, Δ = 43.8 ms) using the well-known gaussian phase distribution approximation for diffusion within spheres2,9. The ADCIC was calculated for various different values of the intrinsic cytosolic diffusivity, ranging in [1; 3] µm2/ms.
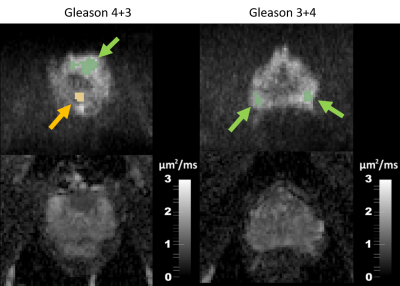
In parallel, corresponding benign and malignant ROIs were segmented on MRI and a clinical mono-exponential ADCMRI was fit with b = [0; 1500] s/mm2 (Figure 2), which was then assumed to be the weighted average of IC and EES contributions:
$$\mathrm{ADC_{MRI}\approx f_{IC} ADC_{IC}+(1-f_{IC}) ADC_{EES}}$$
Statistical Welch’s t-test was performed looking for differences in properties among malignant and benign tissues.
Results
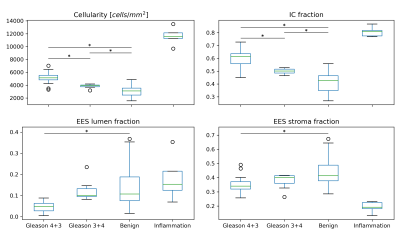
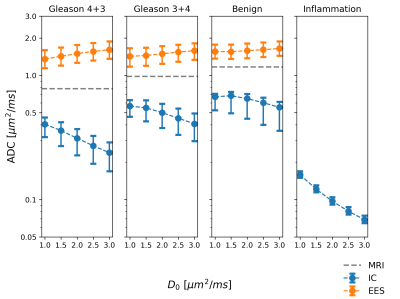
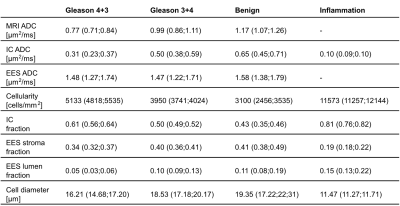
In Table 1 and Figure 3, a summary of the findings is given with median and inter-quartile range values. A significantly lower ADCMRI was observed in malignant compared to benign imaging ROIs (p<0.001 for both Gleason 4+3, 3+4). In parallel, estimated ADCIC from histology was also found to decrease with higher tissue malignancy (p<0.001 for both Gleason 4+3, 3+4). This can be seen in Figure 4, which shows the histology-derived ADCIC and the ADCEES contribution with respect to the ADCMRI. Increasing IC fraction was also found (p<0.001, p=0.004 for Gleason 4+3 and 3+4 respectively) and tissue cellularity (p<0.001, p=0.02, respectively) compared to benign tissue. Lower lumen and stroma fractions were found for Gleason 4+3 over benign tissue (p<0.001 and p=0.002), but not for Gleason 3+4. Significant differences were also found for Gleason 4+3, with lower ADCMRI, lower ADCIC, higher IC fraction and higher cellularity, as compared to Gleason 3+4 (p<0.001).Discussion
A higher tissue malignancy had been previously correlated with decreasing ADC2,3,10,11, due to the presence of water-restrictive epithelial cells and lower stromal and lumen spaces10. In accordance, we have found low ADCIC and low lumen fraction in the histology of the analysed malignancies.Furthermore, the histology-derived ADCIC along with the IC fraction both seem to be the main drive of the measured ADCMRI towards lower values, as compared to changes in ADCEES. In small areas of inflammation with highly packed cells, ADCIC appears even lower, reflecting highly- restricted water diffusion. That might cause clinical ADCMRI to be misinterpreted as malignant tissue, as an overlap in ADC values of prostatitis and prostate cancer has been reported12,13. Additionally, the microstructure differences between Gleason 3+4 and Gleason 4+3 malignancies could be captured by clinical ADCMRI and ADCIC, reflecting the different histological properties, which points at the development of diffusion MRI for clinical diagnosis and evaluation, potentially differentiating benign and malignant tissues before undergoing invasive surgical procedures.
Conclusion
In this work we have shown that co-localized histology and clinical ADC properties allow to obtain microstructure information of benign and two different malignant prostate cancer types, further distinguishing them. These findings highlight the clinical value of dMRI and illustrate the potential of informing clinical ADC measurements with data from co-localised histology, when available. Moreover, our results could also serve as unique reference for further studies focusing on validation and modelling explicitly the multi-component nature of the dMRI signal in prostate.Acknowledgements
AGR was funded by the European Association for Cancer Research (Travel Fellowship #765). RPL was supported by CRIS Foundation (TALENT19-05), Instituto de Salud Carlos III (PI18/01395), Prostate Cancer Foundation and Fero Foundation. FG received support of a fellowship from ”la Caixa” Foundation (ID 100010434), fellowship: LCF/BQ/PR22/11920010. EP was supported by EPSRC EP/N021967/1, and by Prostate Cancer UK, Targeted Call 2014, Translational Research St.2, grant number PG14-018-TR2. SS received support from EPSRC (EP/S021930/1).References
1. Ackerman JJ, Neil JJ. The use of MR-detectable reporter molecules and ions to evaluate diffusion in normal and ischemic brain. NMR Biomed 2010;23(7):725-733.2. Panagiotaki E, Chan RW, Dikaios N, et al. Microstructural characterization of normal and malignant human prostate tissue with vascular, extracellular, and restricted diffusion for cytometry in tumours magnetic resonance imaging. Invest Radiol 2015;50(4):218-227.
3. Gupta RT, Kauffman CR, Garcia-Reyes K, et al. Apparent Diffusion Coefficient Values of the Benign Central Zone of the Prostate: Comparison With Low- and High-Grade Prostate Cancer. AJR Am J Roentgenol 2015;205(2):331-336.
4. Bourne R, Panagiotaki E. Limitations and Prospects for Diffusion-Weighted MRI of the Prostate. Diagnostics (Basel) 2016;6(2).
5. Bourne RM, Bailey C, Johnston EW, et al. Apparatus for Histological Validation of In Vivo and Ex Vivo Magnetic Resonance Imaging of the Human Prostate. Front Oncol 2017;7:47.
6. Panagiotaki E, Ianus A, Johnston E, Chan R, Atkinson D, Alexander D. Optimised VERDICT MRI protocol for prostate cancer characterisation. Proceedings of the 23rd meeting of the International Society for Magnetic Resonance in Medicine. Volume 2872: International Society for Magnetic Resonance in Medicine 2015.
7. Bankhead P, Loughrey MB, Fernandez JA, et al. QuPath: Open source software for digital pathology image analysis. Sci Rep 2017;7(1):16878.
8. Grussu F, Bernatowicz K, Casanova-Salas I, et al. Diffusion MRI signal cumulants and hepatocyte microstructure at fixed diffusion time: Insights from simulations, 9.4T imaging, and histology. Magn Reson Med 2022;88(1):365-379.
9. Bailey C, Collins DJ, Tunariu N, et al. Microstructure Characterization of Bone Metastases from Prostate Cancer with Diffusion MRI: Preliminary Findings. Front Oncol 2018;8:26.
10. Chatterjee A, Watson G, Myint E, Sved P, McEntee M, Bourne R. Changes in Epithelium, Stroma, and Lumen Space Correlate More Strongly with Gleason Pattern and Are Stronger Predictors of Prostate ADC Changes than Cellularity Metrics. Radiology 2015;277(3):751-762.
11. Manetta R, Palumbo P, Gianneramo C, et al. Correlation between ADC values and Gleason score in evaluation of prostate cancer: multicentre experience and review of the literature. Gland Surg 2019;8(Suppl 3):S216-S222.
12. Nagel KN, Schouten MG, Hambrock T, et al. Differentiation of prostatitis and prostate cancer by using diffusion-weighted MR imaging and MR-guided biopsy at 3 T. Radiology 2013;267(1):164-172.
13. Yu J, Fulcher AS, Turner MA, Cockrell CH, Cote EP, Wallace TJ. Prostate cancer and its mimics at multiparametric prostate MRI. Br J Radiol 2014;87(1037):20130659.
Figures