0316
THE ADDED VALUE OF HYBRID MULTIDIMENSIONAL MRI TO MULTIPARAMETRIC MRI IN DIAGNOSING CLINICALLY SIGNIFICANT PROSTATE CANCERS1RADIOLOGY, University of Chicago, Chicago, IL, United States, 2BIOSTATISTICS, University of Chicago, Chicago, IL, United States
Synopsis
Keywords: Prostate, Quantitative Imaging, Multiparametric MRI
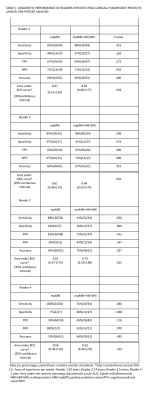
1. In a retrospective review of 61 men by four readers, two inexperienced-readers had higher accuracy using multiparametric MRI+Hybrid-Multidimensional MRI (mpMRI+HM-MRI)=82%,81% versus Multiparametric MRI=77%, 71% (p=.006,<.001) and higher specificity=89%,88% versus 84%,75% (p=.009, p<.001) on a per-sextant analysis.
2. Multiparametric MRI+Hybrid-Multidimensional MRI increased trainee specificity=46% versus Multiparametric MRI=7% (p<.001) on a per-patient analysis.
3. Multiparametric MRI+Hybrid-Multidimensional MRI increased interreader Kappa agreement=0.36 versus Multiparametric MRI (mpMRI) Kappa=0.17 (p=0.009) in diagnosing clinically significant prostate cancer (CS PCa).
INTRODUCTION
Hybrid multidimensional MRI (HM-MRI) combines T2 relaxation and diffusion and calculates tissue estimates based on interdependently-measured ADC and T2 values, producing volume fractions of the luminal, epithelial and stromal ADC and T2 values. In cancers, densely packed and highly proliferative malignant epithelial cells replace normal stroma and luminal space, resulting in lower lumen and stromal fractions and higher epithelial fractions than benign tissue (1-3). Hybrid Multidimensional MRI (HM-MRI) fraction measurements were validated with quantitative histology results from whole mount prostatectomy and against evaluation of expert pathologists (4,5,6). In another observer study, HM-MRI as a standalone tool was shown to be effective in detecting prostate cancer (7). However, the optimal results may be obtained by the combination of HM-MRI along with conventional mpMRI. The purpose of our study was to evaluate HM-MRI as a tool to enhance radiologists' performance in diagnosing clinically significant prostate cancer and improving interreader agreement.MATERIALS AND METHODS
In this retrospective study, 61 patients underwent 3.0-T MRI with a six-channel cardiac phased-array coil-endorectal coil combination. The HM-MRI sequence, acquired prior to mpMRI, consisted of multiple combinations of echo times and b-values with a spin-echo module and diffusion-sensitizing gradients placed symmetrically about the 180° pulse followed by a single-shot echo-planar imaging readout. Compartmental analysis of HM-MRI signals were modeled as unmixed pools of water in three tissue components: stroma, epithelium, and lumen. Tissue composition was calculated on a voxel-by-voxel basis using a three-compartment model by fitting the following equation, described in previously published papers (4,5,6,7):S/So=∑(n=1)(n=3)▒〖Vn×exp〗(−〖ADC〗n×b−TE/〖T2〗n)
where Vn, T2n, and ADCn are the volume fractions, T2 and ADC values for each tissue [stroma, epithelium, and lumen] compartment, S is the signal intensity at each combination of echo time and b-value, S0 is the signal intensity at the lowest echo time and b-value. Cancer regions, identified as conjoint voxels with a minimum 2D size of 25 mm2 with more epithelium (>40 %) and less lumen (<20 %) using the HM-MRI tool, were superimposed in red over the ADC maps using Matlab. This predicted cancer map was displayed as an additional sequence(8). Four readers (Reader 1-4, 1-20 years prostate MRI experience), retrospectively reviewed the mpMRI, followed by the mpMRI+HM-MRI in the same sitting. Readers were blinded to clinical information and pathologic results. In phase 1, mpMRI PIRADS positive score of 3-5 (based on PIRADS v2.1) and lesion location were recorded. In Phase 2, the HM-MRI cancer-map was viewed with mpMRI, with lesions and locations rescored. Suspected cancer lesions were matched with histologically-confirmed prostate cancer (Gleason grade>/=3+4) from 12-core MRI-TRUS biopsy results or prostatectomy whole-mount slices based on the consensus of a radiologist, pathologist and medical physicist. The area under the ROC curve (AUC) and accuracy were the primary endpoints; sensitivity, specificity, positive and negative predictive values were the secondary endpoints. Fleiss Kappa for interreader agreement was calculated on a per-patient and per-sextant basis. Per-tumor analysis was unable to be performed in this retrospective analysis, as pathologic results of the suspected areas not biopsied could not be determined.
RESULTS
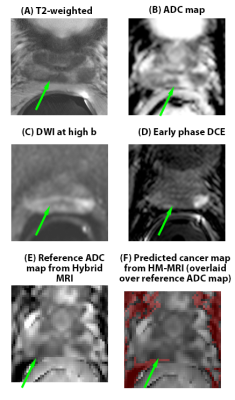
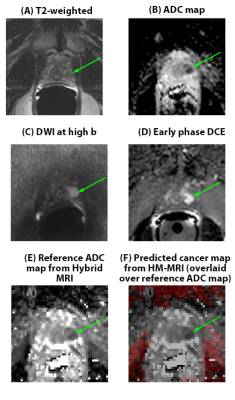
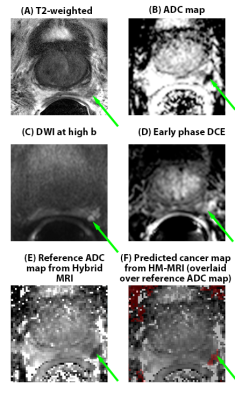
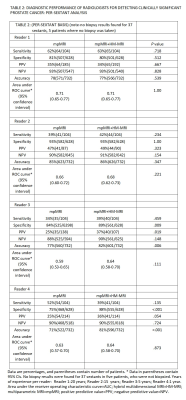
34 patients had clinically significant cancer (>/= Gleason 3+4), and 27 patients had no clinically significant cancer. Of the clinically significant cancers, 19 were Gleason 3+4, 9 were Gleason 4+3, 4 were Gleason 4+4, and 2 were Gleason 4+5. In per-patient analysis (Table 1), inexperienced-readers’ specificity using mpMRI+Hybrid MRI was higher=56%, 48% versus mpMRI alone 33%, 7% (p=.004, <.001), approaching expert-readers’ specificity. In per-sextant analysis (Table 2), the inexperienced-readers’ specificity using mpMRI+Hybrid MRI was higher=89%, 88% versus mpMRI alone=84%,75% (p=.009,<.001). The inexperienced-readers’ mpMRI+HM-MRI PPV (=37%, 36% vs.=25%, 25% (p=.019, .054)); and mpMRI+HM-MRI accuracy were higher (=82%, 81% vs. 77%, 71% (p=.006,<.001)). Figure 1 shows R3/4 mpMRI false negative. No significant improvements were seen for the highly-experienced readers or for other parameters for the inexperienced-readers. Analysis of the subset of per-sextant-prostatectomy patients (n=25), confirmed higher trainee’s mpMRI+HM-MRI specificity=88% versus 74% (p=.004). Figure 2 shows R4 mpMRI false negative. Per-patient mpMRI+HM-MRI Fleiss’ Kappa was higher=0.36(0.26-0.46) versus 0.17(0.07-0.27) (p=.009). Figure 3 shows R1-4 agreement.DISCUSSION
Our study proved mpMRI+HM-MRI increased the inexperienced-readers’ accuracy and specificity and in diagnosing clinically significant prostate cancer, improving interreader agreement in contrast to numerous quantitative and CAD studies reporting higher sensitivity (9-14). Our study’s increased specificity was compromised only for the trainee’s lower sensitivity. Sensitivity and negative predictive value decreased for the trainee-reader, due to the larger resolution of the HM-MRI causing false negatives of lesions smaller than 25 mm2 and inherent cancer-volume underestimation of ADC (15); the HM-MRI map can be optimized to detect smaller cancers. Higher resolution and refinement in transition zone fractional composition limits could improve cancer detection. Additional DWI-artifact-correction tools may increase HM-MRI map accuracy. The expert-readers’ limited experience with HM-MRI and confidence with mpMRI could potentially account for the unaltered performance with mpMRI+HM-MRI for experienced-readers; the intermediate-reader’s knowledge-base of anatomy and artifacts accounted for increased PPV; the trainee’s greater reliance on mpMRI+HM-MRI without the fund of knowledge accounted for false negatives of subtle cancers.CONCLUSION
The addition of HM-MRI-predicted-cancer maps to conventional mpMRI improved inexperienced-readers’ accuracy and specificity in diagnosing clinically significant prostate cancer, and interreader agreement. Further studies across multiple institutions would validate if HM-MRI improves performance of inexperienced-readers.Acknowledgements
No acknowledgement found.References
1.Johnston E, Bonet-Carne E, Ferizi U, Yvernault B, Pye H, Patel D, Clemente J, Piga W, Heavey S, Sidhu H, Giganti F, O’Callaghan J, ……. Punwani S. VERDICT MRI For Prostate Cancer: Intracellular Volume Fraction versus Apparent Diffusion Coefficient. Radiology 2019; 291:391–397.
2.Panagiotaki E, Chan R, Dikaios N, Ahmed H, O’Callaghan J, Freeman A, Atkinson D, Punwani S, Hawkes D, Alexander D. Microstructural Characterization of Normal and Malignant Human Prostate Tissue With Vascular, Extracellular, and Restricted Diffusion for Cytometry in Tumors Magnetic Resonance Imaging. Investigative Radiology April 2015 - Volume 50 - Issue 4 : 218-227.
3.Hectors S, Said D, Gnerre J, Tewari A, Touli B. Luminal Water Imaging: Comparison With Diffusion-Weighted Imaging(DWI) and PI-RADS for Characterization of Prostate Cancer Aggressiveness. J. MAGN. RESON. IMAGING 2020;52:271–279.
4.Chatterjee A, Bourne R,
Wang S, Devaraj A, Gallan A, Antic T, Karczmar G, Oto A. Diagnosis of Prostate
Cancer with Noninvasive Estimation of Prostate Tissue Composition by Using
Hybrid Multidimensional MR Imaging: A Feasibility Study. Radiology 2018;
287:864–873.
5. Chatterjee A, Mercado C, Bourne RM, et al. Validation of Prostate Tissue Composition by Using Hybrid Multidimensional MRI: Correlation with Histologic Findings. Radiology. 2022; 302(2):368-77.
6. Chatterjee A, Antic T, Gallan AJ, et al. Histological Validation of Prostate Tissue Composition Measurement using Hybrid Multi-dimensional MRI: Agreement with Pathologists’ Measures. Abdom Radiol 47, 801–813 (2022). https://doi.org/10.1007/s00261-021-03371-7
7. Lee GH, Chatterjee A, Karademir I, Engelmann R, Yousuf A, Giurcanu M, ... & Oto, A. (2022). Comparing Radiologist Performance in Diagnosing Clinically Significant Prostate Cancer with Multiparametric versus Hybrid Multidimensional MRI. Radiology, 211895.
8. Chatterjee A, Mercado C, Bourne RM, et al. Validation of Prostate Tissue Composition by Using Hybrid Multidimensional MRI: Correlation with Histologic Findings. Radiology. 2022; 302(2):368-77.
9.Youn SY, Choi MH, Kim DH, Lee YJ, Huisman H, Johnson, E., ... & Kamen A. (2021). Detection and PI-RADS Classification of Focal Lesions in Prostate MRI: Performance Comparison Between a Deep Learning-based Algorithm (DLA) and Radiologists with Various Levels of Experience. European Journal of Radiology, 142, 109894.
10.Greer MD, Lay N, Shih JH, Barrett T, Bittencourt LK, Borofsky S, Kabakus I, Law YM, Marko J, Shebel H, et al. Computer-aided Diagnosis Prior to Conventional Interpretation of Prostate MpMRI: An International Multi-reader Study. Eur. Radiol. 2018, 28, 4407–4417. [CrossRef] [PubMed]
11. Faiella E, Vertulli D, Esperto F, Cordelli E, Soda P, Muraca RM, Moramarco LP, Grasso RF, Zobel BB, Santucci D, Quantib Prostate Compared to an Expert Radiologist for the Diagnosis of Prostate Cancer on mpMRI: A Single-Center Preliminary Study, Tomography 2022, 8, 2010-2019. https://doi.org/10.3390/tomography8040168
12.Giannini V, Mazzetti S, Cappello G, Doronzio VM, Vassallo L, Russo F, Giacobbe A, Muto G, Regge D. Computer-Aided Diagnosis Improves the Detection of Clinically Significant Prostate Cancer on Multiparametric-MRI: A Multi-Observer Performance Study Involving Inexperienced Readers. Diagnostics 2021, 11, 973. https:// doi.org/10.3390/diagnostics11060973
13.Gaur S, Lay N, Harmon SA, Doddakashi S, Mehralivand S, Argun B, Barrett T, Bednarova S, Girometti R, Karaarslan E, Kural AR, Oto A, Purysko AS, Antic T, Magi-Galluzzi C, Saglican Y, Sioletic S, Warren AY, Bittencourt L, Fütterer JJ, Gupta RT, Kabakus I, Law YM, Margolis DJ, Shebel H, Westphalen AC, Wood BJ, Pinto PA, Shih JH, Choyke PL, Summers RM, Turkbey B. Can Computer-aided Diagnosis Assist in the Identification of Prostate Cancer on Prostate MRI? A Multi-center, Multi-reader Investigation. Oncotarget. 2018 Sep 18;9(73):33804-33817. doi: 10.18632/oncotarget.26100. PMID: 30333911; PMCID: PMC6173466
14.Hambrock T, Vos PC, De Kaa CAH, Barentsz JO, Huisman H. Prostate Cancer: Computer-aided Diagnosis with Multiparametric 3-T MR Imaging—Effect on Observer Performance. Radiology 2013, 266, 521–530. [CrossRef] [PubMed]
15. Sun C, Chatterjee A, Yousuf A, Antic T, Eggener S, Karczmar GS, Oto A. Comparison of T2-Weighted Imaging, DWI, and Dynamic Contrast-Enhanced MRI for Calculation of Prostate Cancer Index Lesion Volume; Correlation With Whole-Mount Pathology, American Journal of Roentgenology, 212(2), 351-356.
Figures