0309
MUSE reconstruction of multishot DW-EPI is not limited by SNR at 0.5T1Biomedical Engineering, Dalhousie University, Halifax, NS, Canada, 2BIOTIC / Biomedical Translational Imaging Centre, Nova Scotia Health, Halifax, NS, Canada, 3Physics and Atmospheric Science, Dalhousie University, Halifax, NS, Canada, 4Synaptive Medical, Toronto, ON, Canada, 5Diagnostic Radiology, Dalhousie University, Halifax, NS, Canada, 6Diagnostic Radiology, Nova Scotia Health, Halifax, NS, Canada, 7BIOTIC / Biomedical Translational Imaging Centre, IWK Health, Halifax, NS, Canada
Synopsis
Keywords: Low-Field MRI, Susceptibility, Susceptibility distortion
Multi-shot echo planar imaging (EPI) can be used to reduce spatial distortion and improve spatial resolution in diffusion-weighted imaging; however, patient motion between shots can result in large intra-shot phase differences and increased artifact. It is unknown whether multiplexed sensitivity encoding (MUSE) can be applied to reduce these phase differences in DW-EPI images acquired at low field due to the inherently lower SNR. In this work we evaluate the performance of MUSE in DW-EPI data acquired at 0.5T, in terms of SNR and ghost-to-noise ratio (GNR) as a function of signal averaging, and demonstrate that multi-shot DW-EPI is feasible.Introduction
Spatial fidelity in diffusion weighted imaging (DWI) is diminished by geometric distortion due to susceptibility-induced field gradients (SFG), particularly in anatomical areas with air-bone interfaces [1]. One method for reducing SFG distortion is to use a lower applied magnetic field strength, since it is established in the literature that SFG distortion is proportionally reduced at lower field strengths [2]. Alternatively, multi-shot echo planar imaging (EPI) can be used with DWI to reduce SFG-induced phase errors and improve spatial resolution. However, using a multi-shot DW-EPI acquisition requires motion correction, since any patient motion between shots can result in large intra-shot phase differences. Multiplexed sensitivity encoding (MUSE) [3], developed as a motion-corrected multi-shot expansion of sensitivity encoding (SENSE) parallel imaging [4] can be applied to reduce these phase differences. It is unknown whether multi-shot DW-EPI can benefit from SFG distortion reduction at low-field because of the inherent reduced SNR, which may lead to inaccurate MUSE estimation of phase. However, modern signal processing techniques and gradient and RF hardware may provide enough noise reduction to yield sufficient signal-to-noise ratio (SNR), particularly if used with acquisition sequences and reconstruction techniques that minimize spatial distortion. In this study, we evaluated MUSE reconstruction of DW-EPI at low-field for increased robustness to susceptibility distortion. We present a comparison of field strengths and explore the potential use of MUSE for improved image quality and artifact reduction. We further investigate the impact of increasing the number of signal averages on overall SNR and ghost-to-noise ratio (GNR).Methods
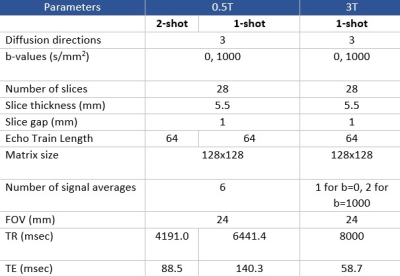
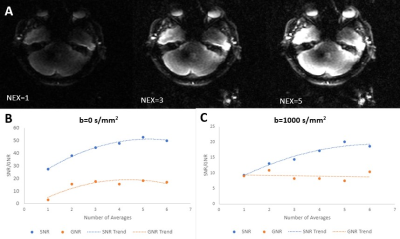
A 0.5T MRI system from Synaptive Medical was used to investigate low-field SNR limits in MUSE. This device has a head-only design with conformable 16 channel receive coil for improved SNR and is effective for rapid EPI because of its strong, fast gradients with a maximum strength of 100 mT/m and slew of 400T/m/s [5]. DWI on this system has been demonstrated previously to be comparable to clinical systems [6]. A healthy volunteer was scanned at 0.5T under an REB-approved protocol using an axial EPI-DWI acquisition sequence, and re-scanned at 3T (MR750, GE Healthcare) for comparison. Parameters were kept consistent within reason across field strengths (Figure 1). A matched slice traversing the paranasal sinuses and internal auditory canal was chosen from both image sets. 3T and standard 0.5T images were reconstructed with their respective built-in reconstruction pipelines. In the raw 0.5T data, signal averages were kept separate to be combined offline as desired, allowing exploration of the impact of signal averaging. MUSE image reconstruction was performed in Matlab (R2020a) with the three necessary components of Cartesian-gridded k-space, estimated phase maps, and coil sensitivities according to the method described in [3]. Slice-specific coil sensitivities for each of the 16 receive channels were obtained. Cartesian gridded k-space for each shot (with inherent Nyquist ghosting artifacts) was obtained from the raw data prior to any combination across diffusion direction, average, or channel. Low-resolution phase maps were derived from the total variance of approximate full-FOV SENSE-reconstructions according to [7] and applying [8]. The calculated phase variance was applied to the coil sensitivity maps to obtain pseudo-sensitivities describing the phase differences between shots. Finally, the inverted phase-corrected sensitivity matrix was used to construct a full FOV motion-corrected image. To investigate the relationship between SNR and GNR with increasing number of combined signal averages at 0.5T (up to six), regions of interest (ROIs) were drawn on b=0 s/mm2 and b=1000 s/mm2 images in areas of uniform noise, temporal lobe, and ghosting. The mean of the signal and ghost ROIs were divided by the standard deviation of the noise to determine SNR and GNR respectively.Results and Discussion
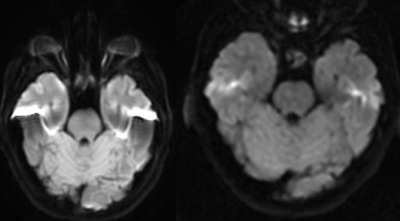
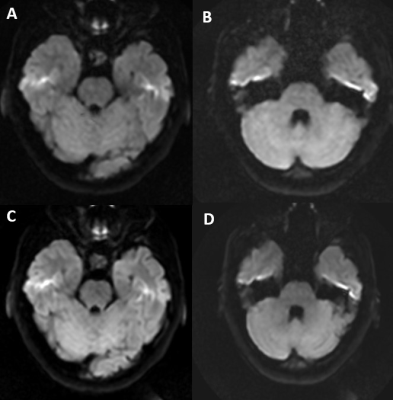
DW-EPI was visually compared at 3T and 0.5T for SFG distortion around air-bone interfaces (Figure 2). Qualitative reduction in signal hyperintensity associated with distortion is observed at 0.5T on the posterior temporal lobe corresponding to the internal auditory canal. Distortion is similar across 1- and 2-shot acquisitions at 0.5T (Figure 3). MUSE reconstruction was performed with up to six combined averages (Figure 4a). Qualitative increases in signal, noise, and ghosting were observed with increased averaging. Further quantitative comparison across averages at b=0 s/mm2 revealed SNR and GNR ranges from 27.3 to 52.6 and 3.0 to 18.3, respectively (Figure 4b). At b=1000 s/mm2, SNR and GNR ranged from 9.3 to 20.1 and 7.5 to 10.9, respectively (Figure 4c). A both b-values, SNR and GNR do not scale proportionally, suggesting that the ghosting artifact may behave more as noise than as signal, and hence does not increase at the same rate as a function of number of averages.Conclusions and Future Work
This study presents a low-field DW-EPI acquisition with MUSE reconstruction for reduced susceptibility distortion. Sufficient SNR of 20 [9] was produced with a single average and was demonstrated to increase as expected with additional averaging. These results suggest that the raw SNR of DW-EPI at 0.5T remains above the level needed for MUSE phase correction. This implies that multi-shot DW-EPI may be possible at 0.5T for improved imaging in clinically relevant areas of SFG, such as characterization of cholesteatoma.Acknowledgements
This work was supported by a grant from the Natural Sciences and Engineering Research Council as well as a Dalhousie Faculty of Engineering Graduate Award. Research on the 0.5T magnet is supported by an INOVAIT Focus Fund grant with matching funding provided by Synaptive Medical. Thanks to Jeff Stainsby of Synaptive Medical for work on the offline reconstruction and helpful discussions.References
[1] J.-P. Vercruysse, B. de Foer, M. Pouillon, T. Somers, J. Casselman, and E. Offeciers, “The value of diffusion-weighted MR imaging in the diagnosis of primary acquired and residual cholesteatoma: a surgical verified study of 100 patients.,” Eur Radiol, vol. 16, no. 7, pp. 1461–7, Jul. 2006, doi: 10.1007/s00330-006-0160-2.
[2] H.-M. Klein, “Low-Field Magnetic Resonance Imaging,” Fortschr Röntgenstr, vol. 192, pp. 537–548, 2020.
[3] N. kuei Chen, A. Guidon, H. C. Chang, and A. W. Song, “A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE),” Neuroimage, vol. 72, pp. 41–47, May 2013, doi: 10.1016/j.neuroimage.2013.01.038.
[4] K. P. Pruessmann, M. Weiger, M. B. Scheidegger, and P. Boesiger, “SENSE: Sensitivity encoding for fast MRI,” Magn Reson Med, vol. 42, no. 5, pp. 952–962, Nov. 1999, doi: 10.1002/(SICI)1522-2594(199911)42:5<952::AID-MRM16>3.0.CO;2-S.
[5] J. A. Stainsby et al., “Imaging at 0.5 T with high-performance system components System Components.”
[6] J. A. Stainsby, C. T. Harris, G. A. Bindseil, C. N. Wiens, P. J. Beatty, and A. T. Curtis, “High-Performance Diffusion Imaging on a 0.5T System.”
[7] M. Chiew, “SENSE Parallel Imaging,” https://users.fmrib.ox.ac.uk/~mchiew/docs/SENSE_tutorial.html, 2022.
[8] M. Lourakis, “TV-L1 Image Denoising Algorithm.” MATLAB Central File Exchange, 2022. [Online]. Available: https://www.mathworks.com/matlabcentral/fileexchange/57604-tv-l1-image-denoising-algorithm
[9] R. S. Owen and F. W. Wehrli, “Predictability of SNR and reader preference in clinical MR imaging,” Magn Reson Imaging, vol. 8, no. 6, pp. 737–745, 1990.
Figures