0307
Ultra-Low Field Quantitative T2 Mapping1Physics and Astronomy, University of British Columbia, Vancouver, BC, Canada, 2Hyperfine Inc., Guilford, CT, United States, 3CaliberMRI Inc., Boulder, CO, United States, 4Radiology, University of British Columbia, Vancouver, BC, Canada, 5Department of Neuroimaging, King’s College London, London, United Kingdom, 6Maternal, Newborn & Child Health Discovery & Tools, Bill and Melinda Gates Foundation, Seattle, WA, United States, 7Department of Medical Radiation Physics, Lund University, Lund, Sweden, 8Medicine (Neurology), University of British Columbia, Vancouver, BC, Canada
Synopsis
Keywords: Low-Field MRI, Relaxometry
We demonstrated quantitative T2 mapping at 0.064 T using a multi-echo spin-echo sequence and CALIPR subspace constrained image reconstruction. CALIPR reconstruction results had minimal evidence of undersampling, providing better artifact suppression and preservation of fine anatomical detail than compressed sensing reconstruction.
Phantom validation work was performed using 14 vials doped with known concentrations of MnCl2 and showed a relationship between measured T2 and MnCl2 concentration in agreement with expectations from a mathematical model. In-vivo, we demonstrated short acquisition time (8m:40s or 5m:59s), strong agreement with a highly-sampled reference acquisition, and T2 maps with good contrast between tissue types.
Introduction
With reduced cost, simplicity of operation and negligible installation requirements, ultra-low-field systems are poised to drastically increase the accessibility of MRI. Previous work has pioneered the development of relaxation time mapping at ultra-low field1,2. However, inherently low SNR can lead to long acquisition times and limit the utility and accuracy of T2 mapping in-vivo.In this work we develop a rapid T2 mapping protocol on a point-of-care, ultra-low field MRI system that does not compromise on SNR or T2 map quality. This is accomplished by combining a multi-echo spin-echo pulse sequence with a low-rank reconstruction approach previously introduced for multi-component T2 mapping at high field3.
Methods
All data was acquired on a 64mT Hyperfine Swoop System (hardware version 1.7, software rc8.5.0).Acquisition
A T2 mapping acquisition was developed with three versions:
1) “Reference”: for comparison (3D multi-echo fast spin echo, FOV=180x220x180 mm3, resolution=3x3x5 mm3, 120 echoes, echo spacing=4.1 ms, TR=1500 ms, k-space undersampling factor=1.0, partial Fourier=0.7 in both the phase and slice directions, acquisition time=33m:35s),
2) “Accelerated 120 Echo”: identical to the Reference but with k-space undersampling factor=4.0 (acquisition time=8m:40s)
3) “Accelerated 80 Echo”: identical to the Accelerated 120 Echo version but with 80 echoes instead of 120 and TR=1250ms instead of 1500ms (acquisition time=5m:59s).
Reconstruction
Image reconstructions were performed offline in Matlab (R2019b) using BART4. Reconstructions were performed using non-uniform fast Fourier transform (nuFFT), compressed sensing (CS), as well as the constrained, adaptive, low-dimensional, intrinsically precise reconstruction (CALIPR) framework3, a recently introduced approach for subspace constrained reconstructions. ESPIRiT was used for coil sensitivity map estimation5. CS and CALIPR reconstructions were solved using FISTA6 on GPU (NVIDIA Titan RTX) with L1 wavelet regularization factors of λ=0.002 and λ=0.005 for phantom and in-vivo data, respectively. All CALIPR reconstructions used a fixed subspace size of 8.
Phantom Experiment
The Accelerated 120 Echo sequence was acquired in a phantom (Mini Hybrid Phantom Model 137, serial number CMRI 137-0002, CaliberMRI, Inc., Boulder, CO USA), containing 14 separate vials with known concentrations of MnCl2. The behaviour of T2 relaxation times as a function of MnCl2 concentration $$$ c $$$ should follow:
$$ \frac{1}{T_{2}(c)} = \frac{1}{T_{2}(c=0)} + Rc $$
for some unknown T2 of distilled water at 0.064 T, $$$ T_{2}(c=0) $$$, and nuclear relaxivity $$$ R $$$.
Regions of interest were manually delineated in the centre of each vial, from which mean and standard deviation T2 times were extracted. Because the true T2 times of the vials at 0.064 T are unknown, $$$ T_{2}(c) $$$ data was fit to this model to assess whether our results were consistent with this known model.
In-Vivo Experiments
All three versions of the T2 mapping sequence were acquired for a single healthy volunteer. Default T2w and FLAIR anatomical images were acquired for the same subject in another session.
Note that apart from Figure 1, all T2 mapping results used CALIPR reconstructions.
Results
Compared to nuFFT and CS reconstructions with the same Accelerated 80 Echo dataset (Figure 1), the CALIPR images and T2 maps showed minimal evidence of undersampling, with better artifact suppression and preservation of fine anatomical detail.For the phantom experiment in Figure 2, the T2 map showed the expected trend in T2 times between vials, based on the known concentrations of MnCl2. With appropriate scaling, all the vials could be distinguished visually by their T2 times. Our measured T2 as a function of MnCl2 concentration agreed with the expected model (Figure 3).
Figure 4 showed excellent agreement between the Reference, Accelerated 120 Echo, and Accelerated 80 Echo in-vivo datasets reconstructed using the CALIPR framework. Compared to the Reference sequence, the Accelerated results show only subtle loss of detail and do not appear to introduce a bias in the estimated T2 values.
In Figure 5, T2w and FLAIR anatomical images are supplemented with our in-vivo T2 map to demonstrate an example protocol that can be achieved with an ultra-low field system.
Discussion
Despite the inherently lower signal-to-noise ratio, some aspects of ultra-low field MRI physics are uniquely favourable to T2 mapping. Negligible specific absorption rate limitations allow for long echo trains of closely spaced 180° refocusing pulses. Shorter T1 relaxation times at ultra-low field do not require as long an effective TR (TR-TEmax) to recover the signal, which boosts SNR efficiency by minimizing dead time in T2-weighted spin echo sequences.Noise robustness of the CALIPR framework reconstruction is especially apparent at later echo times, where nuFFT and CS reconstructions show artifacts resulting from low signal, due to T2 decay.
Our T2 estimation in the phantom vials with the lowest MnCl2 concentrations have large standard deviations suggestive of imprecise T2 estimation. This variability is unsurprising, as T2 >> TEmax for these vials and the temporal sampling is insufficient to characterize these long T2 components. However, our current echo train is sufficient to characterize most brain tissue, which has T2 on the order of 100 ms at 0.064 T1.
Conclusion
Here we developed an approach for performing rapid, in-vivo T2 mapping on ultra-low field MRI systems. Despite rapid acquisition (6-9 minutes), this technique achieved sufficiently high SNR to characterize a wide range of T2 times in phantom and distinguish brain tissue types in-vivo.Acknowledgements
We thank Hyperfine Inc for their involvement and support. FP, RPAGT, MEP, MH, and SR are employed by Hyperfine Inc. EL, SW and SHK received funding from the Bill & Melinda Gates Foundation. This research is additionally funded by Natural Sciences and Engineering Research Council of Canada (AVD - Alexander Graham Bell Canada Graduate Scholarship, SHK- Grant RGPIN-2018-03904) and Michael Smith Healthcare BC (SHK).References
1 O’Reilly, T. & Webb, A. G. In vivo T1 and T2 relaxation time maps of brain tissue, skeletal muscle, and lipid measured in healthy volunteers at 50 mT. Magnetic Resonance in Medicine 87, 884-895 (2022).2 Jordanova, K. V. et al. Quantitative MRI measurements for T1 and T2 at 0.064T. In Proceedings of the 31st Annual Meeting of ISMRM (2022).
3 Dvorak, A. V. et al. The CALIPR framework comprehensively improves acquisition, reconstruction & analysis of multi-component relaxation imaging. In Proceedings of the 31st Annual Meeting of ISMRM (2022).
4 Uecker, M. The Berkeley Advanced Reconstruction Toolbox (BART). DOI: 10.5281/zenodo.592960. doi:10.5281/zenodo.592960 (2019).
5 Uecker, M. et al. ESPIRiT--an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med 71, 990-1001, doi:10.1002/mrm.24751 (2014).
6 Beck, A. & Teboulle, M. A Fast Iterative Shrinkage-Thresholding Algorithm for Linear Inverse Problems. Siam Journal on Imaging Sciences 2, 183-202, doi:10.1137/080716542 (2009).
7 Bloembergen, N. & Morgan, L. Proton relaxation times in paramagnetic solutions. Effects of electron spin relaxation. The Journal of Chemical Physics 34, 842-850 (1961).
Figures
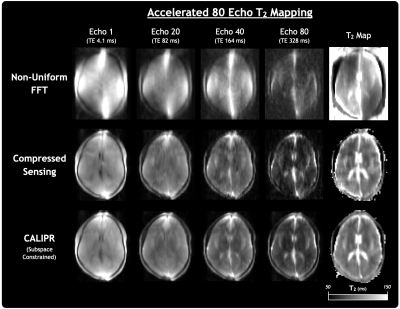
Figure 1: Comparison of results from 1) non-uniform fast Fourier transform (FFT), 2) compressed sensing, and 3) constrained, adaptive, low-dimensional, intrinsically precise reconstruction (CALIPR) reconstruction of the Accelerated 80 Echo T2 mapping sequence. Echo images are labelled with their echo time (TE).
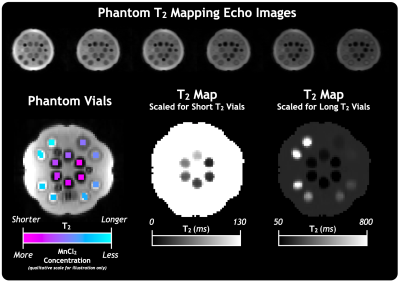
Figure 2: Images and quantitative maps for the Accelerated 120 Echo sequence with CALIPR reconstruction in phantom. The 14 phantom vials are doped with a range of high to low MnCl2 concentration, resulting in a range of short to long T2 relaxation times. The same T2 map is shown with two scales, to better depict the variation between the vial relaxation times.
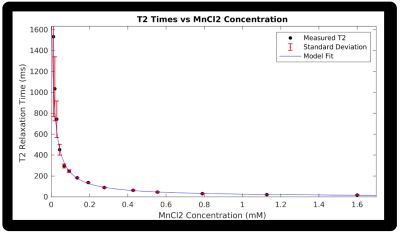
Figure 3: T2 relaxation times as a function of MnCl2 concentration for 14 phantom vials, for the Accelerated 120 Echo sequence with CALIPR reconstruction. The data fit to the expected model is also shown, along with error bars representing the standard deviation of voxel T2 values within each vial.
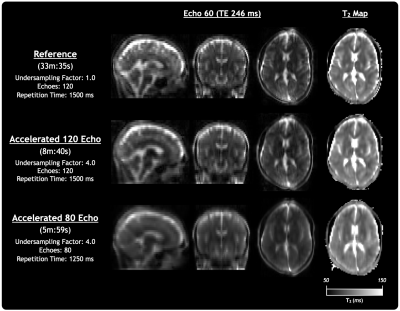
Figure 4: Comparison of echo images and quantitative T2 maps from the Reference, Accelerated 120 Echo, and Accelerated 80 Echo sequences acquired in-vivo and reconstructed with CALIPR.
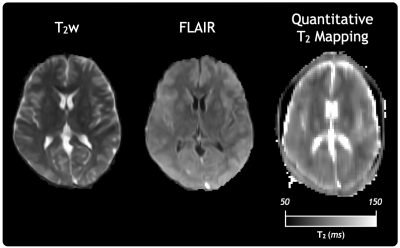
Figure 5: Example ultra-low field MRI protocol with T2w and FLAIR anatomical images, as well as a quantitative T2 map acquired in a separate session for a healthy volunteer. T2 mapping data was acquired using the Accelerated 80 Echo sequence and reconstructed with CALIPR.