0293
Diffusion Bubble Model: A Novel Method For Detecting Neuroinflammation in Mouse Brain With Sanfilippo Syndrome1Institute of Biomedical Engineering, Polytechnique Montreal, Montreal, QC, Canada, 2CHU Sainte-Justine University Hospital Center, University of Montreal, Montreal, QC, Canada, 3NeuroPoly Lab, Institute of Biomedical Engineering, Polytechnique Montreal, Montreal, QC, Canada, 4Cerebral Imaging Centre, Douglas Research Center, McGill University, Montreal, QC, Canada, 5Centre Hospitalier Universitaire Sainte-Justine Research Center, University of Montreal, Montreal, QC, Canada, 6Department of Anatomy and Cell Biology, McGill University, Montreal, QC, Canada, 7Department of Computer Engineering and Software Engineering, Polytechnique Montreal, Montreal, QC, Canada, 8Department of Pharmacology, University of Montreal, Montreal, QC, Canada
Synopsis
The Diffusion Bubble Model (DBM) is a new method for detecting neuroinflammation in the brains of mice with Sanfilippo Syndrome. This rare and debilitating disorder primarily affects children and causes progressive neurodegeneration. DBM utilizes diffusion spectrum derived from dMRI to detect brain injuries such as inflammation. It can work in both white and gray matter with limited number of diffusion directions. The study found that brain injuries with inflammation had reduced the fraction of the slow diffusion component and increasing the fraction of the fast diffusion component. The findings may establish non-invasive biomarkers for detecting and evaluating neuroinflammation diseases.
Introduction
Mucopolysaccharidosis IIIC (MPSIIIC, Sanfilippo syndrome type C) is a rare and debilitating lysosomal storage disorder that primarily affects children, causing progressive neurodegeneration. Mice models of model of MPS IIIC produced in Dr Pshezhetsky laboratory, the knockin Hgsnat P304L mouse (KI mouse) and the Hgsnat-Geo strain (Knockout mice), present signs of significant neuroinflammation and synaptic defects, though more important in the KI mice 1,2. Compared wild type and knock-out counterparts, the KI mouse presented a higher increase in microglia and GFAP+ astrocytes in different regions of the brain (hippocampi, somatosensory cortex) coinciding with the presence of inflammatory cytokines (Glucosamine amends CNS pathology in mucopolysaccharidosis IIIC mouse expressing misfolded HGSNAT - PMC (nih.gov)). Recently, slow and fast diffusion fractions derived from dMRI have been utilized to detect brain injuries such as edema, inflammation. Available model requires time-consuming multi-shell data acquisition, or highly complex reconstruction algorithms 3,4. In this study, we present a novel simple model that can be used to detect slow and fast diffusion fraction changes in MPSIIIC brains, meant to work in both white and gray matter with a limited number of diffusion directions. Aim: To develop a new method to detect neuroinflammation of MPSIIIC animals. Hypothesis: Brain injuries with inflammation will cause edema, and axonal injury, thus reducing the fraction of the slow diffusion component and increasing the fraction of the fast diffusion component.Methods
Two groups of 7-month mice were chosen and imaged on a 7T Bruker MRI scanner. 10 mice control group (WT); 8 mice MPSIIIC group (KI mice). After perfusion (4% PFA, 5h) and brain removal, brains were imaged in a custom-built syringe with a solenoid coil: 2D spin-echo sequences, one b0 and 25 b-values (0 < b ≤ 3000s/mm2 ) with different b-vectors, field of view 12mm × 12mm, resolution 0.15 × 0.15 × 0.4mm3 , and TR/TE: 3300ms/32ms.Diffusion data were processed by denoising, resampling, and registration. Then, a novel model, Diffusion Bubble Model (DBM), was used to decompose the diffusion-weighted signals (DWIs). It treated DWIs as a sum of diffusion tensors with equal axis lengths and residual errors. A least-square method was used for the decomposition for the diffusion spectrum. Written the equation into matrix format, S = WF , the solution is F̂ = (W T W + αI)−1 W T Ŝ. Here, α is used to avoid overfitting; I is identity matrix; F̂ is the diffusion fraction array (diffusion spectrum).
Four ROIs, cortex, hippocampus, corpus callosum, caudoputamen region (atlas name), were chosen on the T2w template. Diffusion spectrum and DTI results of two groups were plotted for comparison (Mann-Whitney test p <0.05).
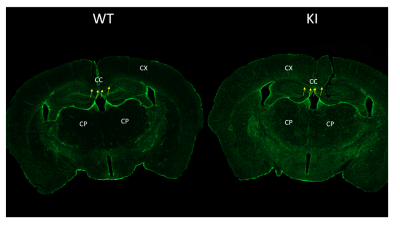
Wild type and KI mice brain floating cryosections were immunostained for astrocytes using GFAP antibody followed by fluorescent secondary antibody. Images were scanned in a Axioscan (Zeiss) .
Results
14 components with increasing diffusivity level were used. Comparing KI animals to control animals: In the cortex: fractions of two slow diffusion components were decreased significantly (−6.82/%, p < 0.01; −4.24/%, p < 0.02 ); fractions of two fast diffusion components were increased significantly (+22.92/%, p < 0.01; 110.61/%, p < 0.04). DTI results also showed increased AD, RD and MD. In the hippocampus: fractions of two slow diffusion components were decreased significantly ( −14.05/%, p < 0.01; −9.89/%, p < 0.01 ); fractions of three fast diffusion components were increased significantly (+31.73/%, p < 0.01; +76.94/%, p < 0.01,+145.40/%, p < 0.02). DTI results also showed increased AD, RD and MD and decreased FA. In the corpus callosum, DBM results displayed one reduced slow diffusion fraction and one increased fast components fraction. In caudo-putaminal region, DTI showed increased AD, RD and MD. While DBM showed only one slow diffusion components eduction. Preliminary results show increased GFAP expression in the KI mouse compared to the control mouse, as in previous publications.Discussion
In all four chosen ROIs, DTI results showed increased AD, RD, MD. As reported 5 microarchitectural destruction edema and gliosis. Accordingly, the diffusion spectrum showed a reduced fraction of slow diffusion (intracellular) components and an increased fraction of fast diffusion components.Conclusion
Our novel method detected Sanfilippo syndrome type C injuries diffusivity changes in the corpus callosum cortex, hippocampus, corpus callosum and caudoputaminal region. These findings may establish non-invasive biomarkers for detecting and evaluating neuroinflammation diseases.Acknowledgements
No acknowledgement found.References
[1] X. Pan, M. Taherzadeh, et al, “Glucosamine amends CNSpathology in mucopolysaccharidosis IIIC mouse expressing misfolded HGSNAT,” J. Exp. Med., vol. 219, Aug.2022.
[2] C. Pará, P. Bose, et al, “Early defectsin mucopolysaccharidosis type IIIC disrupt excitatory synaptic transmission,” JCI Insight, vol. 6, Aug. 2021.
[3] Y. Wang, Q. Wang, et al, “Quantification of increased cellularity during inflammatory demyelination,” Brain, vol. 134, pp. 3590–3601,Dec. 2011.
[4] N. S. White, T. B. Leergaard, et al, “Probing tissue microstructure withrestriction spectrum imaging: Histological and theoretical validation,” Hum. Brain Mapp., vol. 34, pp. 327–346,Feb. 2013.
[5] J. H. Kim, D. N. Loy, et al, “Noninvasive diffusion tensorimaging of evolving white matter pathology in a mouse model of acute spinal cord injury,” Magn. Reson. Med.,vol. 58, pp. 253–260, Aug. 2007.
Figures