0292
Short-term changes in brain structure, perfusion and oxygen metabolism in young adults infected with Omicron: A case control study using MRI1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2Department of Radiology, the Affiliated BenQ Hospital of Nanjing Medical University, Nanjing, China, 3College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, China, 4Department of Biomedical Engineering, School of Medicine, Johns Hopkins University, Baldimore, MD, United States
Synopsis
We investigated the short-term changes in brain structure, perfusion and oxygen metabolism in young adult patients infected with SARS-COV-2 Omicron but with mild symptoms in China using multimodal MRI. The clinical and brain MR imaging characteristics were compared between patient group (n=98) and healthy control group(n=50). Our findings suggest that there are no significant short-term changes in brain structure, perfusion and oxygen metabolism determined by multimodal MR imaging in young adult patients infected with SARS-CoV-2 Omicron. The potential trend of decline in the volume of cerebral nucleus in the patient group needs further investigation.
Introduction
The pandemic of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to a global health emergency. The clinical symptoms of patients infected with SARS-COV-2 variate among different variants1. Previous studies have shown that infection with SARS-CoV-2 is associated with neurological symptoms during and after the acute phase of illness2-4. MRI studies reported that patients infected with SARS-CoV-2 Alpha, Beta and Gamma showed decline in the volume of whole brain5 and increases in blood brain barriers (BBB) permeability6. However, it is unclear if patients infected with SARS-COV-2 Omicron suffer from damages in neurological system. This study aimed to investigate the short-term changes in brain structure, perfusion and oxygen metabolism in young adult patients infected with SARS-COV-2 Omicron but with mild symptoms in China using multimodal MRI.Methods
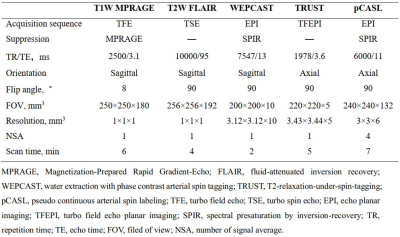
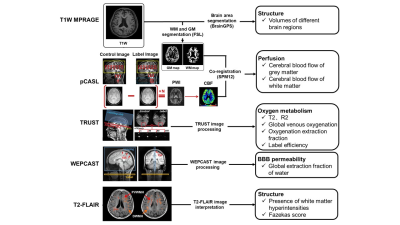
Study population: Young adult patients recently infected with SARS-COV-2 Omicron were consecutively recruited. The inclusion criteria included: (1) age ranged from 20 to 30 years; (2) SARS-COV-2 positive results in antigen or nucleic acid test <2 months; and (3) non-hospitalized mild symptoms during infection. Patients with brain diseases or contraindications to MR examination were excluded. Age and sex-matched asymptomatic subjects were included as healthy control (patients: healthy controls = 2:1). All recruited subjects underwent brain MR imaging. The study protocol was approved by institutional review board and written consent form was obtained from all subjects. MR imaging protocol: Multimodal MR imaging protocol (Table 1) for brain structural, perfusion, and oxygen metabolism was conducted on a whole-body 3.0T MR scanner (Ingenia TX, Philips Healthcare, Best, The Netherlands) with 32-channel head coil. MR image processing and interpretation: The volume of different brain structure regions was measured on T1 weighted MR images by using in-house MATLAB (MathWorks, Natick MA) scripts and Brain Geodesic Positioning System (Brain GPS, Anatomy Works, LLC). To quantify the cerebral perfusion images with the pixel-wise cerebral blood flow (CBF), SPM toolkit and in-house MATLAB scripts were utilized7. The global venous oxygenation and brain oxygen extraction fraction (OEF) were measured at the superior sagittal sinus on MR images8. The extraction fraction of water (E) was calculated to determine the global blood-brain-barrier permeability and the details of processing can be found in Lin et al9, 10. White matter hyperintensities (WMHs) were identified and graded using Fazekas scores11 by one experienced observer blinded to clinical information. The detailed processing workflow was presented in Figure 1. Statistical analysis: The clinical characteristics, volume of different brain regions, MR imaging measurements of blood-brain-barrier (BBB) permeability, cerebral blood flow and WMHs between patients and healthy controls were compared using independent t test, Mann Whitney U test, or Chi-square test when appropriate with SPSS 26.0.Results
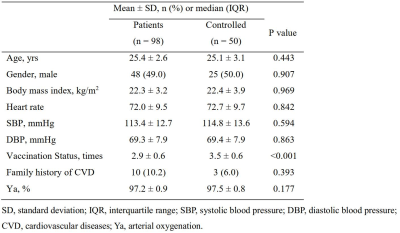
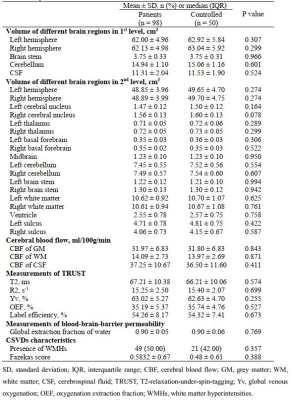
A total of 98 patients (mean age: 25.4±2.6 years; 48 males) infected with SARS-COV-2 Omicron and 50 healthy controls (mean age: 25.1±3.1 years; 50 males) were included. The clinical characteristics are summarized in Table 2. The comparisons of brain structure, perfusion and oxygen metabolism between patients and healthy controls were listed in Table 3. Patients infected with SARS-COV-2 Omicron showed significantly smaller number of vaccination compared to health controls (2.9±0.6 vs. 3.5±0.6, P<0.001). There was marginally significant difference in the volume of right cerebral nucleus between patient group and healthy control group (P=0.078). No significant differences were found in the volumes of other brain regions (P>0.05). There were no significant differences in CBF of gray matter (31.97±6.83 ml/100g/min vs. 31.80±6.83 ml/100g/min, P=0.843), white matter (14.09±2.73 ml/100g/min vs. 13.97±2.69 ml/100g/min, P=0.871) and cerebrospinal fluid (37.25±10.67 ml/100g/min vs. 36.50±11.60 ml/100g/min, P=0.411) between two groups. No significant differences can be found in OEF (35.19±5.37% vs. 35.74±4.76%, P=0.527) and E (0.90±0.05 vs. 0.90±0.06, P=0.769) between two groups as well.Discussion and Conclusion
In this study, we found that participants in the controlled group had more vaccination times than patient group. This finding suggests that more effective vaccination may improve the protection of human immunity, which has been found in a previous study by Siripongsatian et al12. Although there was no significant difference in the volumes of all brain regions between patient and healthy control groups, a trend of decline in the volumes of right cerebral nucleus in patient group was found. Our findings are in accordance with a study by Heine et al, which can be explained by the fact that infection with SARS-CoV-2 Omicron was associated with persistent fatigue 13. Although a study by Rastogi et al reported that SARS-CoV-2 may induce loss of BBB permeability14, there was no significant difference in global water extraction fraction between patient and healthy control groups in the present study. These results can be explained by the protection of vaccination and the decreased toxicity of Omicron15. In the future, more detailed brain regions need to be considered and it will be valuable to follow-up the patients infected with SARS-CoV-2 Omicron to determine the long-term changes. In conclusion, there are no significant short-term changes in brain structure, perfusion and oxygen metabolism determined by multimodal MR imaging in young adult patients infected with SARS-CoV-2 Omicron. The potential trend of decline in the volume of cerebral nucleus in the patient group needs further investigation.Acknowledgements
None.References
1. World Health Organization. COVID-19 disease in children and adolescents. published September 29, 2021. Published online 2021. https://apps.who.int/iris/rest/bitstreams/1375120/retrieve.
2. Divani AA, Andalib S, Biller J, et al. Central Nervous System Manifestations Associated with COVID-19. Curr Neurol Neurosci Rep. 2020;20(12):60.
3. Méndez R, Balanzá-Martínez V, Luperdi SC, et al. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J Intern Med. 2021;290(3):621-631.
4. Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615.
5. Douaud G, Lee S, Alfaro-Almagro F, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697-707.
6. Yang RC, Huang K, Zhang HP, et al. SARS-CoV-2 productively infects human brain microvascular endothelial cells. J Neuroinflammation. 2022;19(1):149.
7. Chen Z, Zhang X, Yuan C, Zhao X, van Osch MJP. Measuring the labeling efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med. 2017;77(5):1841-1852.
8. Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-relaxation-under-spin-tagging MRI. Magn Reson Med. 2008;60:357-363.
9. Lin Z, Li Y, Su P, et al. Non-contrast MR imaging of blood-brain barrier permeability to water. Magn Reson Med. 2018;80(4):1507-1520.
10. Lin Z, Jiang D, Liu D, et al. Noncontrast assessment of blood-brain barrier permeability to water: Shorter acquisition, test-retest reproducibility, and comparison with contrast-based method. Magn Reson Med. 2021;86(1):143-156.
11. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351-356.
12. Heine J, Schwichtenberg K, Hartung TJ, et al. Structural brain changes in patients with post-COVID fatigue: a prospective observational study. EClinicalMedicine. 2023;58:101874.
13. Rastogi A, Bingeliene A, Strafella AP, Tang-Wai DF, Wu PE, Mandell DM. Reversible neurological and brain MRI changes following COVID-19 vaccination: A case report. J Neuroradiol. 2022 Nov;49(6):428-430.
14. Siripongsatian D, Kunawudhi A, Promteangtrong C, Kiatkittikul P, Jantarato A, Choolam A, Ponglikitmongkol K, Siripongboonsitti T, Kaeowirun T, Chotipanich C. Alterations in 18F-FDG PET/MRI and 15O-Water PET Brain Findings in Patients With Neurological Symptoms After COVID-19 Vaccination: A Pilot Study. Clin Nucl Med. 2022 Mar 1;47(3):e230-e239.
15. Lapointe HR, Mwimanzi F, Cheung PK, et al. Serial infection with SARS-CoV-2 Omicron BA.1 and BA.2 following three-dose COVID-19 vaccination. Front Immunol. 2022;13:947021.