0289
Using Diffusion-weighted MR spectroscopy to quantify age- and inflammation-associated changes in glial morphology in-vivo1CUBRIC, Cardiff University, Cardiff, United Kingdom, 2CISC, Brighton and Sussex Medical School, Brighton, United Kingdom, 3ICM-Paris Brain Institute, Paris, France
Synopsis
Age-associated cognitive decline and neurodegenerative disorders have been linked to altered immune function and inflammation, and particularly to the role of microglia. Current methods for imaging (micro)glia in humans are limited to TSPO-PET, a costly and invasive technique with poor cellular specificity. Here we acquired diffusion weighted (DW) MRS in 15 young and 15 older participants, each scanned twice, after Interferon-1beta or placebo. Results supported our prior hypotheses of selective effects of Interferon-1beta on the apparent diffusion coefficient of thalamic Choline (p<0.040), providing support for DW-MRS as a novel in-vivo method for quantifying glial morphology.
Introduction
Neuroinflammation is increasingly implicated in the aetiology of human psychiatric and neurodegenerative disorders and age-associated cognitive decline1. During systemic inflammation microglia undergo a temporary change in shape and function, releasing inflammatory proteins that can disrupt the function of surrounding neurons. Recent data suggest that diffusion-weighted magnetic resonance spectroscopy (DW-MRS) may be sensitive to systemic endotoxin-induced changes in glial morphometry2. We combined an Interferon-beta-1b (IFN-b) challenge model with DW-MRS to measure inflammation-induced changes in the apparent diffusion coefficients (ADC) of glial and neuronal metabolites in young and older healthy participants. Interferons are a family of cytokines known to trigger a mild transient inflammatory response. We hypothesised: (a) IFN-b would induce a significant increase in the apparent diffusion coefficient (ADC) of choline (Cho, glial) but not N-acetyl-aspartate (NAA, neuronal); (b) Age will selectively modulate effects of inflammation on choline (glial) ADC; (c) Age will be associated with lower relative concentration of NAA and higher relative concentration of Cho.Methods
Using a within-subject study design, fifteen young (6 male, mean 25.2+/-5.1 years) and 15 older (5 male, mean 62.6 +/- 4.1 years) participants received a subcutaneous injection of either IFN-b (100 μg) or placebo in two separate sessions. Blood pressure, heart rate, temperature and behavioural data were monitored hourly. Blood samples were collected at baseline, 4 and 6½ hours post-injection for differential cell count, cytokine and transcriptomic analysis.MRI/MRS data were obtained 5 hours after each injection. An MPRAGE reconstructed in 3 orthogonal planes was used to position two 4.5 cm3 DW-MRS volumes of interest (VOIs) on the left thalamus and left corona radiata (20 x 15 x 15 mm3 voxel size). We used a bipolar DW-MRS sequence based on a semi-LASER sequence with 4 diffusion weightings (b=0 s/mm2 and 3 b=3500 s/mm2 with diffusion gradients in three orthogonal directions). Spectral analysis was performed using a customised HSVD script written in Matlab; the values obtained from the script for the three orthogonal gradient directions at high b value were averaged and the resulting mean values were used to compute the ADC values for the three metabolites. The relative concentrations we computed as ratios to creatine, based on the HSVD estimates from the spectra acquired at b=0. The main effect of interferon and age, as well as their interaction were tested for metabolites ADC and concentration.
Results
Main effect of interferon: we observed significant condition (IFN-b/placebo) x time interactions in heartrate (+11/ -5.1 beats-per-minute), body temperature (+1.02/+0.3 C), sickness (+4.1/+ 1.6 points), negative mood (+6.3/+2.5 points) and systemic neutrophil, monocyte and lymphocyte counts (all p<0.01) confirming robust peripheral immune and central sickness responses. Paired t-test showed a significant increase in Choline diffusivity in the thalamic VOI only following IFN-b compared to placebo (t(28)=-2.15, p=0.40) (Figure 1). The ADC of NAA and Creatine (Cr) did not significantly differ between conditions in either grey or white matter regions. Furthermore, no significant differences in the metabolites’ relative concentration were found in either region between conditions.Correlation with circulating cytokines: This IFN-b-induced change in Choline ADC was significantly associated with IFN-b-induced increases in plasma interleukin-6 (IL-6, a cytokine produced in response to inflammation) measured 6½ hours post- baseline (r(29)=0.37,p<0.005) (Figure 2).
Age associated effects: In the placebo condition, we found significantly lower thalamic NAA ADC in the older compared to the younger group (t(27) = 2.86, p=0.008) (Figure 3). Older age was also associated with significantly lower NAA relative concentration (tNAA/tCr) in both grey (t(17.54)= 2.49, p=0.023) and white (t(27)= 2.94, p=0.007) matter VOIs (Figure 4) and significantly higher Choline relative concentration (tCho/tCr) in white matter (t(27)=-2.23, p = 0.034) (Figure 5).
Discussion
Our primary finding was an increase in thalamic Cho ADC after IFN-b compared to placebo, which correlated with IFN-b induced changes in the circulating cytokine IL-6. This finding is in line with evidence from animal models where glia undergo acute activation-associated cytomorphological changes during systemic inflammatory challenge1. Mice DW-MRS studies using Myo-inositol (Ins) and Cuprizone models of inflammation have reported elevated Ins and Cho ADCs which also correlated with histological measures of inflammation including changes in astrocytic and microglial area fractions3. Furthermore, in humans, studies on neurological diseases such as systemic lupus erythematosus and ALS have also found elevated diffusivities in choline and creatine4, 5.We also observed age-associated differences in the relative concentrations of NAA (neurons) and choline (glia) with older individuals showing lower NAA and higher Cho relative concentrations compared to their younger counterpart consistent with human and rodent data showing higher glial and lower neuronal densities in older individuals9, 10, 11. Finally, we also identified lower thalamic NAA diffusivity in older adults. Evidence from the literature shows that the main morphometric measures of neurons (total volume, dendritic surface area, dendritic length and diameter, spine number and density) significantly decrease in healthy ageing, which may lead to decreased neuronal space for metabolite diffusion, and potentially reduce the ADCs 6, 7,8.
Conclusion
Our findings show that DW-MRS was able to detect changes in Cho diffusion associated with IFN-b, confirming and extending previous studies that suggest this technique can quantify glial morphological changes associated with mild inflammatory challenges2.Acknowledgements
No acknowledgement found.References
1. Verkhratsky, A., Parpura, V., Pekna, M., Pekny, M., & Sofroniew, M. (2014). Glia in the pathogenesis of neurodegenerative diseases. Biochemical Society Transactions, 42(5), 1291–1301. https://doi.org/10.1042/BST20140107
2. De Marco, R., Ronen, I., Branzoli, F., Amato, M., Asllani, I., Colasanti, A., Harrison, N. A., Cercignani, M. (2022) Diffusion-weighted MR spectroscopy (DW-MRS) is sensitive to LPS-induced changes in human glial morphometry: A preliminary study. Brain Behav Immun, 99, 256-265
3. Genovese, G., Palombo, M., Santin, M. D., Valette, J., Ligneul, C., Aigrot, M.-S., Abdoulkader, N., Langui, D., Millecamps, A., Baron-Van Evercooren, A., Stankoff, B., Lehericy, S., Petiet, A., & Branzoli, F. (2021). Inflammation-driven glial alterations in the cuprizone mouse model probed with diffusion-weighted magnetic resonance spectroscopy at 11.7 T. NMR in Biomedicine, 34(4), e4480. https://doi.org/10.1002/nbm.4480
4. Ercan, E., Magro-Checa, C., Valabregue, R., Branzoli, F., Wood, E. T., Steup-Beekman, G. M., Webb, A. G., Huizinga, T. W. J., van Buchem, M. A., & Ronen, I. (2016). Glial and axonal changes in systemic lupus erythematosus measured with diffusion of intracellular metabolites. Brain, 139(5), 1447–1457. https://doi.org/10.1093/brain/aww031
5. Reischauer, C., Gutzeit, A., Neuwirth, C., Fuchs, A., Sartoretti-Schefer, S., Weber, M., & Czell, D. (2018). In-vivo evaluation of neuronal and glial changes in amyotrophic lateral sclerosis with diffusion tensor spectroscopy. NeuroImage: Clinical, 20, 993–1000. https://doi.org/10.1016/j.nicl.2018.10.001
6. Bishop, N. A., Lu, T., & Yankner, B. A. (2010). Neural mechanisms of ageing and cognitive decline. Nature, 464(7288), 529–535. https://doi.org/10.1038/nature08983
7. Morrison, J. H., & Hof, P. R. (2003). Changes in cortical circuits during aging. Clinical Neuroscience Research, 2(5), 294–304. https://doi.org/10.1016/S1566-2772(03)00006-9
8. Raz, N., Gunning-Dixon, F., Head, D., Rodrigue, K. M., Williamson, A., & Acker, J. D. (2004). Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiology of Aging, 25(3), 377–396. https://doi.org/10.1016/S0197-4580(03)00118-0
9. Martínez-Pinilla, E., Ordóñez, C., del Valle, E., Navarro, A., & Tolivia, J. (2016). Regional and Gender Study of Neuronal Density in Brain during Aging and in Alzheimer’s Disease. Frontiers in Aging Neuroscience, 8. https://www.frontiersin.org/article/10.3389/fnagi.2016.00213
10. Salas, I. H., Burgado, J., & Allen, N. J. (2020). Glia: victims or villains of the aging brain? Neurobiology of Disease, 143, 105008
11. Robillard, K. N., Lee, K. M., Chiu, K. B., & MacLean, A. G. (2016). Glial cell morphological and density changes through the lifespan of rhesus macaques. Brain, behavior, and immunity, 55, 60-69.
Figures
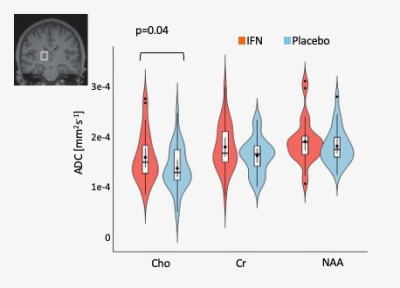
Figure 1. Left Thalamus metabolite distribution of choline, creatine and NAA ADC. P-value represents significant effect of IFN on ADC of choline compared to placebo (Interferon condition is in red, placebo in blue).
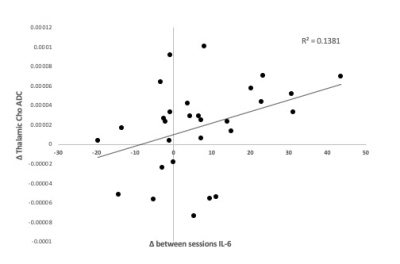
Figure 2. Effects of IFN on IL-6 and relationship with ADC(Cho) change. Correlation between ADC(Cho) change between the two sessions and difference between IL-6 changes (6 ½ hours post- baseline) in the two sessions.
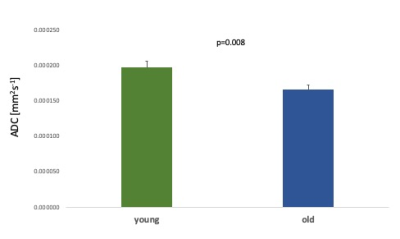
Figure 3. Age-associated differences in thalamic ADC NAA between young and old groups for the placebo condition. Error bars denote the standard error of the mean (SEM).
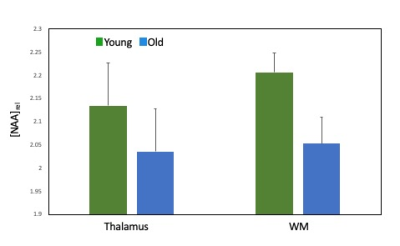
Figure 4. Age-associated differences in thalamic and white matter NAA relative concentrations between young and old groups for the placebo condition. Error bars denote the standard error of the mean (SEM).
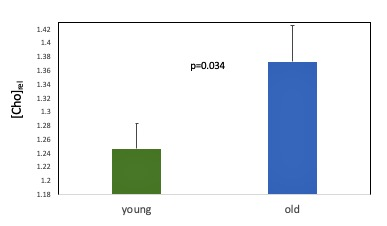
Figure 5. Age-associated differences in the relative concentration of white matter Choline for the placebo condition. Error bars denote the standard error of the mean (SEM).