0285
The sequence of regional structural disconnectivity due to chronic active and inactive multiple sclerosis lesions1Department of Radiology, Weill Cornell Medicine, NYC, NY, United States, 2Department of Neurology, Weill Cornell Medicine, NYC, NY, United States
Synopsis
In this study, we showed that structural disconnectivity due to T2 FLAIR lesions in the ventral attention and subcortical networks, in particular within the supramarginal and putamen, occurs earlier in the sequence of disability (i.e., mild to severe) in multiple sclerosis. We also showed that paramagnetic rim lesion (PRL)-based structural disconnectivity in motor-related regions occurs earlier in the disability event sequence, followed by non-PRL-based structural disconnectivity in the caudate and postcentral gyrus. T2 FLAIR lesion-based structural disconnectivity in subcortical regions, including the thalamus, occurs earliest in the sequence of cognitive impairment (i.e. preserved to impaired).
Introduction
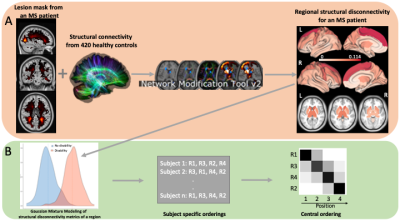
Disease progression can be heterogeneous across people with multiple sclerosis (MS) due in part to varying size, location, and type of brain lesions.1 Moreover, the sequence of the lesions' appearance and their subsequent disruption to white matter streamlines (i.e. structural disconnectivity) make the prediction of disease progression even more challenging. Advanced imaging tools that quantify structural disconnectivity due to MS lesions may enable a better understanding of the impact of lesions' characteristics on disability progression. Discriminative event-based modeling (dEBM) is a data-driven approach that uses cross-sectional data to estimate the sequence of biomarker abnormalities driving disease progression.2,3 Here, we applied dEBM to investigate the sequence of lesion-related regional structural disconnectivity across the spectrum of disability and cognitive impairment in people with MS (See Figure 1).Methods
Four hundred eighty-two people with MS were included. The Expanded Disability Status Scale (EDSS) score was used to classify patients into (i) no or mild disability (EDSS<3) vs (ii) moderate or severe disability (EDSS3) groups. In 363 out of 482 patients, Quantitative susceptibility mapping (QSM) was used to identify lesions with a paramagnetic rim (PRL) representing chronic active lesions. In 171 out of 482 patients, Brief International Cognitive Assessment was used to identify subjects as being cognitively preserved (CP) or impaired (CI). MS lesions were identified using T2 FLAIR MRI. Network Modification tool was used to estimate the resulting regional structural disconnection measures for all representative lesion masks. dEBM was applied to investigate the sequence of regional lesion-based disconnection across disability groups (“no to mild disability” to “moderate to severe disability”) and cognitive status (“CP” to “CI”).Results
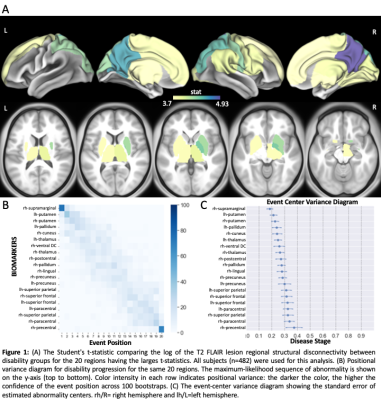
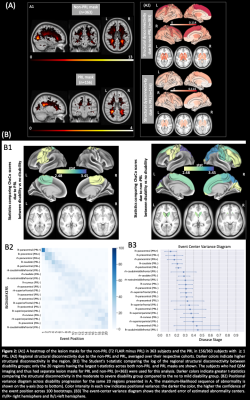
Figure 2 shows the t-statistics of the structural disconnectivity metrics for the 20 regions that were the most significantly different between disability groups in the full cohort of 482 people. The structural disconnectivity metrics were significantly greater, particularly in the bilateral precuneus, right postcentral, and right superior parietal regions in the moderate/severe disability group compared to the no/mild disability group (Fig. 2, A). The EBM results showed that the structural disconnection in the ventral attention and subcortical networks, particularly in the supramarginal and putamen regions, was identified as an early biomarker of moderate or severe disability (Fig. 2, B). Figure 3 shows the heat maps of lesion masks of non-PRL (n=363) and PRL (n=156 subjects with 1 PRL) and their resulting structural disconnectivity maps. PRL tended to cluster in periventricular WM, compared to non-PRLs that are more widespread throughout the WM (Fig. 3, A1). The structural disconnectivity due to non-PRL were greater compared to those due to PRL (Fig. 3, A2). Figure 3 also shows that the earliest biomarkers of disability progression were structural disconnections due to chronic active lesions in the motor-related regions and structural disconnections due to chronic inactive lesions in the subcortical and frontal regions consecutively (Fig. 3, B2). In the smaller group with cognitive testing, the subcortex, particularly in the ventral diencephalon and thalamus regions was identified as an early biomarker of cognitive impairment.Discussion
Our data-driven model revealed that the structural disconnections in the subcortical regions, particularly the thalamus, might be an early biomarker of both disability progression and cognitive decline in MS. Our results further provide new insight into chronic active lesions which appear to be an earlier biomarker, compared to inactive lesions, for disability progression in MS.Conclusion
Such information might be used to identify people with MS who have increased risk of disability progression or cognitive decline in order to provide personalized treatment plans.Acknowledgements
No acknowledgement found.References
1. Barkhof F. The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol. 2002;15(3):239-245.
2. Venkatraghavan V, Dubost F, Bron EE, Niessen WJ, de Bruijne M, Klein S. Event-Based Modeling with High-Dimensional Imaging Biomarkers for Estimating Spatial Progression of Dementia. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). 2019;11492 LNCS:169-180. doi:10.1007/978-3-030-20351-1_13/FIGURES/5
3. Venkatraghavan V, Bron EE, Niessen WJ, Klein S. Disease progression timeline estimation for Alzheimer’s disease using discriminative event based modeling. Neuroimage. 2019;186:518-532. doi:10.1016/J.NEUROIMAGE.2018.11.024
Figures