0284
An investigation of the glial biomarker myo-Inositol as a marker of brain inflammation in elderly patients with and without delirium.
Franklyn A Howe1, Daniel Richardson2, Lauren Binnie3, Uzma Khan4, Philip Rich4, Daniel HJ Davis5, Atticus H Hainsworth2, and Jeremy Isaacs3
1Neurosciences Research Section, St George's, University of London, London, United Kingdom, 2Neurosciences Research Section, St George's, University of London, LONDON, United Kingdom, 3Dept Neurology, St George's University Hospital Foundation Trust, LONDON, United Kingdom, 4Neuroradiology, St George's University Hospital Foundation Trust, LONDON, United Kingdom, 5MRC Unit for Lifelong Health and Ageing, University College London, LONDON, United Kingdom
1Neurosciences Research Section, St George's, University of London, London, United Kingdom, 2Neurosciences Research Section, St George's, University of London, LONDON, United Kingdom, 3Dept Neurology, St George's University Hospital Foundation Trust, LONDON, United Kingdom, 4Neuroradiology, St George's University Hospital Foundation Trust, LONDON, United Kingdom, 5MRC Unit for Lifelong Health and Ageing, University College London, LONDON, United Kingdom
Synopsis
Myo-Inositol is sometimes considered a marker of brain inflammation and studies indicate mI (mI/tCr) increases with age up to the 6th decade. We performed 1H MRS in four brain regions of very elderly hospitalised patients (n=25, aged 67-90 yrs) of which 12 had delirium. Elevated mI/tCr was associated with increasing Fazekas score for white matter damage (p<0.001) in all regions. In addition mI/tCr and mI/NAA showed negative correlation with age (p<0.001). There were no significant differences between patients with or without delirium. Decreased mI/tCr due to reduced glial cell numbers or dysfunction may coincide with increased mI/tCr associated with inflammation.
INTRODUCTION
Delirium affects a significant proportion of elderly hospitalised patients and increases the risk of poor long-term outcome and subsequent dementia, yet the pathophysiology is not well understood. Increased likelihood of delirium has been associated with brain atrophy and white matter hyperintensities (Nitchingham et al 2017) and with plasma markers of inflammation (McNeil et al 2019). Length of delirium duration has been associated with reduced white matter integrity and worsened cognitive outcome in a diffusion MRI study (Morandi et al 2012) and one previous MRS study of adult patients with delirium found alterations in NAA/tCr and tCho/tCr suggestive of neuronal damage and inflammatory processes (Yager et al 2010).In this study we sought to further investigate whether metabolic alterations observed by 1H MRS could provide insight into the inflammatory processes involved in delirium. Of particular interest was measurement of myo-Inositol (Ins), an osmolyte and pre-cursor molecule for membrane synthesis and myelination processes that is predominantly found in glial cells. In normal aging Ins increases slightly with age across different brain regions (Lind et al 2020) and is elevated in a variety of neurodegenerative and inflammatory diseases (Haris et al 2011). Here we performed 1H MRS in four distinct brain regions and used a General Linear Model to assess whether there were global metabolite changes of Ins that could be associated with delirium.
METHODS
Patients aged ≥65 yr admitted to a large teaching hospital, both with and without delirium, were invited to take part in this study. Assent for patients with delirium was acquired via next of kin or senior healthcare personnel in accordance with an approved Ethics protocol. Delirium was confirmed according to DSM-5 criteria and graded using MDAS.MRI included 3D T1w images to assist MRS voxel placement in parietal white matter (PWM), thalamus (THAL), hippocampus (HIPPO) and anterior cingulate cortex (ACC). T1w images were used to quantify Global Cortical Atrophy (GCA) and T2w FLAIR to quantify white matter damage by Fazekas score (1 to 3). 1H MRS was acquired with PRESS using TE 32ms, TR 2000ms and 128-256 averages depending on voxel size. LCModel was used to quantify metabolite signals, with ratios referenced to tCr for Ins, NAA, tCho and Glx across all locations. This provided intracellular metabolite ratios independent of CSF partial volume or of cell density changes due to atrophy or oedema.
A mixed general linear model (GLM) was used to evaluate global patterns of metabolite ratios across all locations and assess for differences between the two patient groups. Age, GCA and Fazekas score were input as covariates. Group comparisons were made with Mann-Witney or Kolmogorov-Smirnov tests.
RESULTS
Data was from n=25 patients with hospitalisation due to infection (n=10), falls (n=6) or other reasons (n=9), mean age 81 +/-6 (range 67 to 90) and for which n=13 had delirium. MRS data was selected for which FWHM < 0.1ppm and SNR >3 (Oz et al 2014), which included all 25 PWM voxels, and in a multi-location subset of 14 patients, comprised data from ACC (n=12), THAL (n=11), and HIPPO (n=8). There were no significant differences in age (p=0.152), Fazekas (p=0.354) and Global Cortical Atrophy (p=0.200) scores between delirium and non-delirium patient groups. Total MDAS was significantly different (p=0.001) between non-delirium (3.3 +/- 0.3) and delirium patients (14.8 +/- 4.9).Figure 1 shows representative spectra from PWM and THAL for a delirium and non-delirium patient, and of note is the greatly reduced Ins peak in the older patient. Figure 2 shows an increase in Ins/tCr ratio with Fazekas score of WM damage in all four locations. GLM analysis (Table 1) indicates a highly significant increase in Ins/NAA and Ins/tCr with Fazekas score, but no difference in these ratios between patient groups. Additionally, there was a significant age-related decrease of Ins/tCr and Ins/NAA that was mostly driven by changes in PWM, THAL and ACC (Figure 3) and also independent of patient group (Figure 4).
DISCUSSION
Elevated Ins/tCr has been positively correlated with increased water diffusion in small vessel disease (SVD) (Nitkunan et al 2006) which is consistent with our results in PWM. However, our results also indicate elevation of Ins/tCr in grey matter regions suggesting there is a global change. Increased Ins with normal aging and injury repair processes have been suggested an inflammatory process of astrocyte activation and proliferation (Harris et al 2015). Our patient group is over a decade older than a previous aging study that reported Ins increases with age in grey and white matter (Lind et al 2020). We believe this the first report of an age-related decrease in Ins. Reduced Ins observed in early traumatic brain injury (Harris et al 2015) and liver disease (Cudalbu et al 2011) has been associated with disturbance of glial cell volume homeostasis. Whether there is a similar mechanistic process in our patients, or there is glial cell loss or dysfunction is not yet clear.In conclusion, a global brain increase in Ins/tCr appears associated with inflammation, but is not different between delirium and non-delirium patients. In opposition to this there is decreased Ins/tCr with age that may be due to glial dysfunction.
Acknowledgements
Funding: Alzheimer’s Research UK and Wellcome Trust (ISSF grant – 204809/Z/16/Z).References
- Cudalbu C et al. Brain Edema in Chronic Hepatic Encephalopathy. Journal of Clinical and Experimental Hepatology. 9:362–382; 2019Haris M et al. In vivo mapping of brain myo-inositol. NeuroImage 54:2079–2085; 2011
- Harris JL et al. Probing astrocyte metabolism in vivo: proton magnetic resonance spectroscopy in the injured and aging brain. Front. Aging Neurosci. 7:202; 2015
- Lind A et al. Regional Myo-Inositol, Creatine, and Choline Levels Are Higher at Older Age and Scale Negatively with Visuospatial Working Memory: A Cross-Sectional Proton MR Spectroscopy Study at 7 Tesla on Normal Cognitive Ageing. The Journal of Neuroscience. 40:8149–8159; 2020
- McNeil JB et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLOS ONE 14:e0226412; 2019
- Morandi A et al. The Relationship between Delirium Duration, White Matter Integrity, and Cognitive Impairment in Intensive Care Unit Survivors as Determined by Diffusion Tensor Imaging. CritCareMed. 40:2182–2189; 2012.
- Nitchingham A et al. A systematic review of neuroimaging in delirium: predictors, correlates and consequences. Int J Geriatr Psychiatry 11:1458-1478; 2017.
- Nitkunan A et al. Correlations between MRS and DTI in cerebral small vessel disease. NMR Biomed. 19:610-6; 2006
- Oz G et al. Clinical Proton MR Spectroscopy in Central Nervous System Disorders Radiology: 270: 658-679; 2014.
- Yager JR et al. Proton magnetic resonance spectroscopy in adult cancer patients with delirium. PsychiatryRes. 191: 128–132; 2011.
Figures
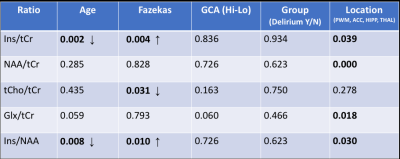
TABLE 1 Results of a general linear model applied to all data to assess for significant differences in metabolite ratios with patient group. Age was a covariate and Patient Group, Fazekas, GCA score (high or low compared to overall median) and MRS voxel location were included as fixed factors. Significant effects are shown as p-values in bold, with arrows indicating the direction of change.
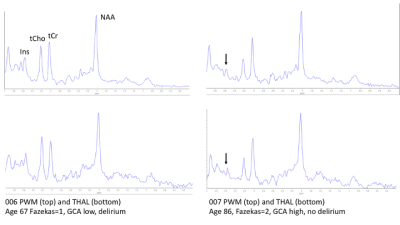
FIGURE 1 Representative spectra from parietal white matter (top) and thalamus (bottom) for: a patient with delirium, age 67, Fazekas=1, and low GCA (left); a patient without delirium age 89, Fazekas=2 and high GCA. The low mI relative to NAA and tCr was a consistent feature associated with oldest patients irrespective of patient group (also see Fig 3). The differences in tCho/tCr ratio between these two patients was not a consistent factor across all the data, nor could it be related to a group difference or other clinical factor.
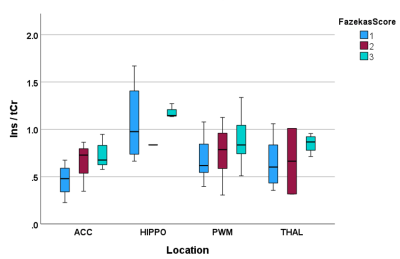
FIGURE 2 Boxplot demonstrating an increase of Ins/tCr ratio with Fazekas score across all locations and that was a feature independent of the patient group.
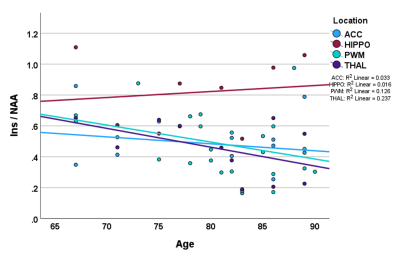
FIGURE 3 The overall inverse correlation of the Ins/NAA ratio with age (and similarly for Ins/tCr) is dominated by changes in the parietal white matter, thalamus and anterior cingulate, and was independent of patient group.
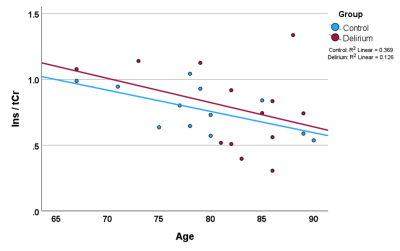
FIGURE 4 Ins/tCr correlates inversely with age independently of group (and similarly for Ins/NAA), as shown for data from parietal white matter for which we had the most MRS data (n=25).
DOI: https://doi.org/10.58530/2023/0284