0263
Radio-pathomic maps of glioblastoma identify phenotypes of non-enhancing infiltration associated with bevacizumab treatment response
Samuel A Bobholz1, Alisha Hoefs1, Jordyn Hamburger1, Allison K Lowman1, Savannah R Duenweg2, Aleksandra Winiarz2, Margaret Stebbins2, Fitzgerald Kyereme1, Jennifer Connelly3, Dylan Coss4, Wade M Mueller5, Mohit Agarwal1, Anjishnu Banerjee6, and Peter S LaViolette1,7
1Radiology, Medical College of Wisconsin, Milwaukee, WI, United States, 2Biophysics, Medical College of Wisconsin, Milwaukee, WI, United States, 3Neurology, Medical College of Wisconsin, Milwaukee, WI, United States, 4Pathology, Medical College of Wisconsin, Milwaukee, WI, United States, 5Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States, 6Biostatistics, Medical College of Wisconsin, Milwaukee, WI, United States, 7Biomedical Engineering, Medical College of Wisconsin, Milwaukee, WI, United States
1Radiology, Medical College of Wisconsin, Milwaukee, WI, United States, 2Biophysics, Medical College of Wisconsin, Milwaukee, WI, United States, 3Neurology, Medical College of Wisconsin, Milwaukee, WI, United States, 4Pathology, Medical College of Wisconsin, Milwaukee, WI, United States, 5Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States, 6Biostatistics, Medical College of Wisconsin, Milwaukee, WI, United States, 7Biomedical Engineering, Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Keywords: Tumors, Tumor
We tested the hypothesis that autopsy-based radio-pathomic maps of glioblastoma pathology reveal distinct phenotypes (hypercellular, hypocellular, hybrid, and well-circumscribed fronts) that differ in patient survival and bevacizumab treatment response. Patients with tumor invasion beyond contrast showed worse survival outcomes compared to patients with well-circumscribed tumors. Additionally, patients with hypocellular components of the non-enhancing front selectively benefit from bevacizumab treatment, with an observable reduction in the hypocellular volume over the course of bevacizumab use.Introduction
Multi-parametric MRI is used to guide treatment delivery and monitor disease progression in glioblastoma patients. However traditional imaging signatures such as T1-weighted gadolinium contrast enhancement and T2-weighted fluid attenuated inversion recovery (FLAIR) hyperintensity are known to be confounded by the presence of treatments such as bevacizumab and other antiangiogenic agents. Our previously developed autopsy-based non-invasive maps of tumor pathology have been used to identify areas of tumor beyond traditional imaging signatures in the post-treated state, which may be able to more precisely monitor treatment response later in a patient’s clinical history. This study used characteristics identified by autopsy tissue-based radio-pathomic maps of tumor pathology to examine heterogenous characteristics of bevacizumab response in glioma patients.Methods
Radio-pathomic models developed in a recently published manuscript were the primary contrast of interest in this study1,2 (see Figure 1 for an overview of the radio-pathomic mapping process). Briefly, pre- and post-contrast T1-weighted images (T1, T1C), T2-weighted FLAIR images, and apparent diffusion coefficient (ADC) images were used as input to predict cellularity, extracellular fluid density, cytoplasm density, and tumor probability using tissue samples aligned to the last clinical imaging prior to death as ground truth. A training dataset of 43 patients was used to train a bagging regression ensemble using 5 by 5 voxel tiles from the MRI as input and voxelwise pathological charact-eristics as labels, with a held-out test set of 22 subjects used for model validation. These models demonstrated good quantitative performance and successfully identified areas of tumor outside the contrast-enhancing region. A system of phenotypes was then developed based on the visual appearance of the non-enhancing tumor front on radio-pathomic maps: Hypercellular Front, where portions of high cellularity extent beyond the contrast-enhancing margin; Hypocellular Front, where portions of low cellularity and high extracellular fluid density extend beyond the contrast-enhancing margin and are thought to indicate areas of edema or hypoxia; Hybrid Front, where both hypocellular and hypercellular regions spread beyond the primary enhancing area, and Well-Circumscribed tumors, where all abnormal pathological findings occur within the contrast-enhancing area (see Figure 2 for examples of each phenotype). Radio-pathomic maps were then generated for an independent dataset of 80 GBM cases with imaging prior to first surgery and graded using the described phenotyping system. Kaplan-Meier analysis was then used to compare differences in survival between phenotypes, as well as patients who had and had not received bevacizumab treatment within each phenotype. Additionally, masks for hyper/hypocellular presence were drawn for a subset of 26 GBM patients imaged over the course of bevacizumab treatment, and linear mixed effect models were used to assess for longitudinal changes in hyper/hypocellular volumes associated with treatment.Results
Figure 3 shows the Kaplan-Meier curves for overall survival within each phenotype. Well-circumscribed patients showed significant/trending increases in survival compared to Hyercellular Front (HR = 2.0, p = 0.05), Hypocellular Front (HR = 2.02, p = 0.03), and Hybrid Front tumors (HR = 1.75, p = 0.09). Figure 4 shows survival curves within patients who have and have not received bevacizumab treatment, divided by phenotype. Only patients with hypocellular or hybrid fronts showed significant survival benefits from bevacizumab treatment (HR=2.35, p=0.02; and HR=2.45, p=0.03, respectively). Figure 5 shows the hypocellular volume per subject with respect to days since initiating bevacizumab treatment. Hypocellular volumes decreased by an average 50.52 mm3 per day of bevacizumab treatment (p=0.002).Discussion
This study investigated radio-pathomic phenotypes of glioblastoma patients to identify differences in patient survival and bevacizumab treatment response. We found that patients with active tumor presence beyond the contrast enhancing region survived less long than patients with tumors well-circumscribed by contrast enhancement, concurring with prior findings observed in our autopsy-based dataset. This suggests that occult glioma invasion may be a hallmark of more aggressive disease, requiring data-driven approaches with rich pathological ground truth to visualize non-invasively. This study also found that patients with hypocellular presence (Hypocellular front and Hybrid front) demonstrate survival benefit from bevacizumab treatment not seen in patients without hypocellular presence. This indicates that these patients may present with pathological features that allow for more efficacious treatment with antiangiogenic agents, and that our non-invasive maps of tumor characteristics could inform clinical decision-making regarding bevacizumab use. Hypocellular regions were also seen to decrease over the course of bevacizumab treatment, further suggesting that these maps may be useful in monitoring antiangiogenic treatment response and underscoring their utility as a response assessment tool for non-enhancing portions of tumor. Future research using this technique may be able to identify subsets of patients within retrospective clinical trials that selectively respond to failed treatments.Acknowledgements
No acknowledgement found.References
1. Bobholz, S. A., Lowman, A. K., Connelly, J. M., Duenweg, S. R., Winiarz, A., Brehler, M., … LaViolette, P. S. (2022). Non-invasive tumor probability maps developed using autopsy tissue identify novel areas of tumor beyond the imaging-defined margin. MedRxiv, 2022.08.17.22278910. https://doi.org/10.1101/2022.08.17.22278910
2. Bobholz, S. A., Lowman, A. K., Brehler, M., Kyereme, F., Duenweg, S. R., Sherman, J., … LaViolette, P. S. (2022). Radio-Pathomic Maps of Cell Density Identify Brain Tumor Invasion beyond Traditional MRI-Defined Margins. AJNR. American Journal of Neuroradiology, 43(5), 682–688. https://doi.org/10.3174/ajnr.A7477
Figures
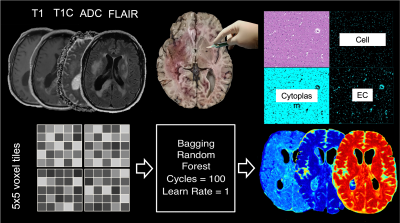
Overview of radio-pathomic map generation. Autopsy samples were aligned to MRI from each patients' last imaging session prior to death and used as ground truth to develop MRI-based models for cellularity, extracellular fluid, and cytoplasm density (see references for further information).
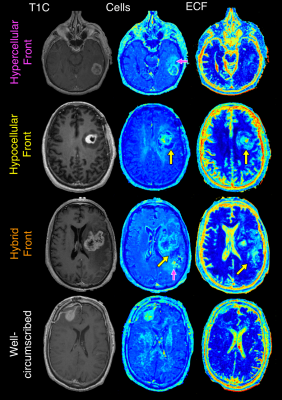
Example patients for each phenotype included in this study.
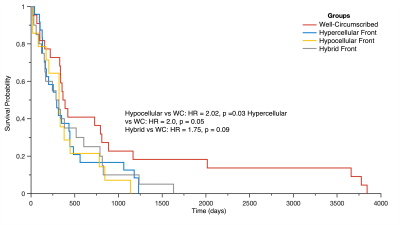
Kaplan
Meier curve assessing survival differences in patients with each phenotype.
Well-circumscribed patients survived the longest, followed by hybrid,
hypercellular, and hypocellular fronts.
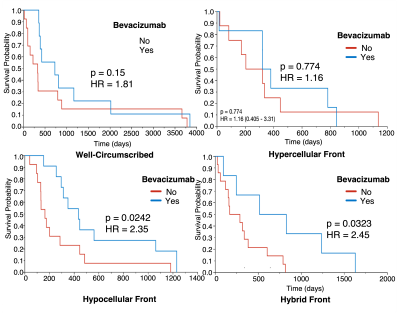
Kaplan
Meier curves assessing survival differences associated with bevacizumab (Bev)
use per phenotype. Only phenotypes with hypocellular presence demonstrated
survival benefit.
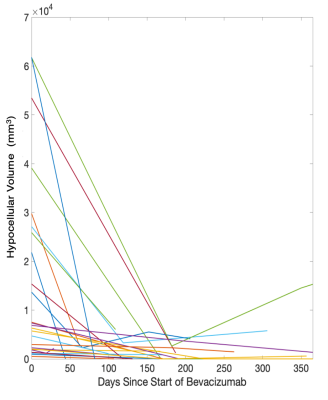
Volume of hypocellular region per subject with respect to time since initiating bevacizumab treatment. Hypocellular
volumes decreased by 50.51 mm3 per
day of bevacizumab use.
DOI: https://doi.org/10.58530/2023/0263