0262
MRE-assessed meningioma stiffness and peripheral adhesion characteristics predict the extent of resectability and recurrence probability
Keni Zheng1, Matthew C. Murphy 1, Emanuele Camerucci 1, Xiang Shan1, Yi Sui1, Armando Manduca 2, Jamie J. Van Gompel3, Richard L. Ehman 1, John III Huston1, and Ziying Yin1
1Radiology, Mayo Clinic, Rochester, MN, United States, 2Physiology and Biomedical Engineering, Mayo Clinic, Rochester, MN, United States, 3Neurosurgery, Mayo Clinic, Rochester, MN, United States
1Radiology, Mayo Clinic, Rochester, MN, United States, 2Physiology and Biomedical Engineering, Mayo Clinic, Rochester, MN, United States, 3Neurosurgery, Mayo Clinic, Rochester, MN, United States
Synopsis
Keywords: Tumors, Brain, Meningiomas, tumor stiffness, tumor adhesion, slip interface imaging, tumor recurrence, tumor resection
This work explored the value of MR elastography (MRE)-measured tumor stiffness and slip interface imaging (SII)-assessed adhesion metrics in predicting the extent of tumor resection and the probability of meningioma recurrence in 52 patients with meningiomas. Tumor adhesion percentage was assessed based on pattern recognition of the normalized octahedral shear strain (NOSS) map. Tumor stiffness was calculated using a neural network-based inversion (NNI). We found that stiffness correlates with the extent of resection (EOR) that was possible at surgery, while the extent of tumor adhesion showed good correlation with tumor recurrence among aggressive tumors with atypical features.Introduction
Meningiomas are the most common primary benign intracranial tumor with an approximate recurrence rate of 35% in Grade II and 73% in Grade III tumors[1]. About 95% of recurrent meningiomas grow in the same location with the same grade or a higher grade than the original tumor. Many efforts have been made to predict meningioma recurrence by evaluating the morphologic, functional, metabolic, or molecular features of the tumors[2-6]. However, the relationships between tumor mechanical properties (such as consistency and adhesion to adjacent structures) and meningioma recurrence and surgical outcomes have not yet been demonstrated. Recently, MR-elastography (MRE) has been increasingly recognized as a useful indicator of meningioma consistency[7]. Slip interface imaging (SII), an MRE-based technique, has used shear strain mapping (normalized octahedral shear strain [NOSS]) to quantify the degree of tumor–peritumoral tissue adhesion in brain tumors[8-10]. This work aimed to explore the predicted value of tumor stiffness and adhesion metrics in meningioma recurrence and extent of resection (EOR) in meningioma patients. Here, tumor adhesion percentage was assessed by a new algorithm based on pattern recognition of the NOSS map, and tumor stiffness was calculated using a neural network-based inversion (NNI)[11,12].Methods
With IRB approval and written informed consent, 52 patients with (1) preoperative MRI/MRE, (2) postoperative MRIs, (3) histopathologically confirmed meningiomas, and (4) tumor size > 2.5 cm were included in this retrospective study. Preoperative MRE was used for tumor stiffness and adhesion analysis. A comparison of pre- and post-operative tumor volumes was used to calculate the EOR: no evidence of residual tumor at post-operative MRI grouped as gross total resection (GTR) and others as non-GTR. Recurrence is defined as the recurrence of a GTR tumor or enlargement of a residual tumor. As atypical features are significant risk factors of recurrence[13], two subgroups were formed: tumors with atypical features (including atypical WHO Grade II and Grade I with atypical features) and tumors with non-atypical features (Grade I without atypical features).All patients had MRE/MRI scans on 3T MR scanners as previously described[10]. The tumor ROI was manually delineated from T1W images registered to MRE space to identify the tumor boundary. NOSS maps were calculated from the measured displacement fields as in[10], and the tumor adhesion percentage was automatically calculated by analyzing the tumor NOSS boundary and characteristics of the neighborhood utilizing pattern recognition. The output measures the % of peritumoral interface length across all tumor-brain interfaces with low shear strain (i.e., adhesion, the white arrow in Fig.1). Tumor stiffness maps were computed using NNI with training data generated as previously described (material parameters randomly assigned, 1-10 kPa for stiffness and 0-0.5 for damping ratio)[11]. Randomly selected mask patches, noise, and phase were applied to each training example to avoid overfitting. The inversion was applied to in vivo data after masking the displacement fields with the tumor ROIs and computing the curl using adaptive methods[12]. Averaged stiffness (kPa) over the tumor ROI was reported. Wilcoxon rank-sum test was used to compare tumor adhesion and stiffness to tumor recurrence and EOR. Fisher's exact test was used for categorical comparison. P<0.05 was considered significant.
Results
The clinical information of the enrolled patients is summarized in Table 1. Ten out of 52 (19.2%) patients had tumor recurrence. There were no differences between the recurrence/non-recurrence groups in terms of basic characteristics, except for the WHO grading and atypical features. Among all tumors with atypical features, the adhesion percentage in the recurrence group was significantly higher than in the non-recurrence group (p=0.04, Fig.2), whereas tumor stiffness showed little difference. The adhesion percentage was also found to be marginally significant between recurrent/non-recurrent groups for tumors with non-GTR (p=0.06, Fig.3). When evaluating all tumors (atypical + non-atypical), we found the tumor adhesion had no correlation with the EOR or recurrence, and tumor stiffness had no correlation with recurrence but showed a trend to distinguish between GTR and non-GTR (p=0.08, Fig.4).Discussion
Our data suggested that for aggressive tumors with atypical features and/or post-operative residuals, recurrent tumors were more adherent than non-recurrent tumors. This may be because tumor adhesion, to some degree, would reflect the extent of invasiveness. The greater the adhesion percentage, the higher the chance that tumor cells will extend beyond the tumor boundary. Interestingly, we did not find any correlation between tumor adhesion and EOR. This may be due to that the current SII analysis focused on tumor-brain parenchyma adhesion, while EOR is largely dependent on the tumor location and surrounding of other critical structures (e.g., nerves and blood supply). High-resolution SII with the capability to assess the adhesion between tumor-critical structural adhesion may address this challenge in the future. Though not significant, we also found that GTR tumors had a higher stiffness compared to non-GTR tumors, which is opposite to previous findings[14]. Future studies are warranted to understand the discrepancies.Conclusion
This study provides preliminary evidence that MRE-measured tumor stiffness can be a good indicator of the extent of successful tumor resection at surgery and that SII-assessed tumor adhesion is promising for predicting the probability of meningioma recurrence. Future studies allowing preoperative identification of high-recurrent meningiomas based on MRE/SII would help considerably with optimal treatment strategies.Acknowledgements
This work was supported by grants from the NIH (R01 EB001981, R61 AT01218, R01 NS113760, and R01 EB027064).References
- Maier H, Ofner D, Hittmair A, Kitz K, Budka H. Classic, atypical, and anaplastic meningioma: three histopathological subtypes of clinical relevance. J Neurosurg 1992;77:616–23.
- Lee JW, Kang KW, Park SH, et al. 18F-FDG PET in the assessment of tumor grade and prediction of tumor recurrence in intracranial meningioma. Eur J Nucl Med Mol Imaging. 2009;36(10):1574-1582.
- Maiti TK, Bir SC, Patra DP, Kalakoti P, Guthikonda B, Nanda A. Spinal meningiomas: clinicoradiological factors predicting recurrence and functional outcome. Neurosurg Focus. 2016;41(2):E6.
- Liu N, Song SY, Jiang JB, Wang TJ, Yan CX. The prognostic role of Ki-67/MIB-1 in meningioma: A systematic review with meta-analysis. Medicine (Baltimore). 2020;99(9):e18644.
- Zhang Y, Chen JH, Chen TY, et al. Radiomics approach for prediction of recurrence in skull base meningiomas. Neuroradiology. 2019;61(12):1355-1364. doi:10.1007/s00234-019-02259-0.
- Mantle RE, Lach B, Delgado MR, Baeesa S, Bélanger G. Predicting the probability of meningioma recurrence based on the quantity of peritumoral brain edema on computerized tomography scanning. J Neurosurg. 1999;91(3):375-383.
- Hughes JD, Fattahi N, Van Gompel J, Arani A, Meyer F, Lanzino G, Link MJ, Ehman R, Huston J. Higher-Resolution Magnetic Resonance Elastography in Meningiomas to Determine Intratumoral Consistency. Neurosurgery. 2015 Oct;77(4):653-8; discussion 658-9.
- Yin Z, Glaser KJ, Manduca A, Van Gompel JJ, Link JM, Hughes JD, Romano A, Ehman RL, Huston J. Slip Interface Imaging Predicts Tumor-Brain Adhesion in Vestibular Schwannomas. Radiology. 2015; 277: 507-517.
- Yin, Z, Hughes JD, Trzasko JD, Glaser KJ, Manduca A, Van Gompel JJ, Link JM, Hughes JD, Romano A, Ehman RL, Huston J.. Slip interface imaging based on MR-elastography preoperatively predicts meningioma–brain adhesion. J Magn Reson Imaging. 2017;46: 1007-1016.
- Yin Z, Lu X, Cohen Cohen S, et al. A new method for quantification and 3D visualization of brain tumor adhesion using slip interface imaging in patients with meningiomas. Eur Radiol. 2021;31(8):5554-5564.
- Scott JM, Pavuluri K, Trzasko JD, et al. Impact of material homogeneity assumption on cortical stiffness estimates by MR elastography. Magn Reson Med. 2022;88(2):916-929.
- Murphy MC, Huston J 3rd, Jack CR Jr, et al. Measuring the characteristic topography of brain stiffness with magnetic resonance elastography. PLoS One. 2013;8(12):e81668. Published 2013 Dec 2.
- Marciscano AE, Stemmer-Rachamimov AO, Niemierko A, et al. Benign meningiomas (WHO Grade I) with atypical histological features: correlation of histopathological features with clinical outcomes. J Neurosurg. 2016;124(1):106-114.
- Itamura K, Chang KE, Lucas J, Donoho DA, Giannotta S, Zada G. Prospective clinical validation of a meningioma consistency grading scheme: association with surgical outcomes and extent of tumor resection [published online ahead of print, 2018 Dec 1]. J Neurosurg. 2018;1-5.
Figures
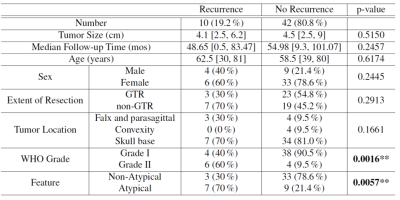
Table.1. The clinical data of meningiomas with and without recurrence
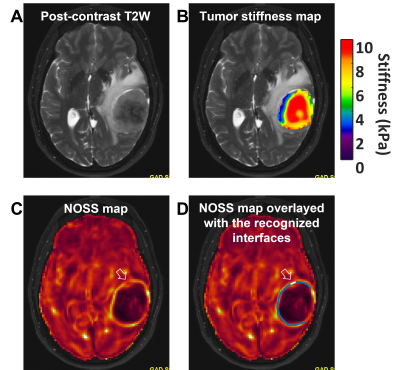
Fig.1.(A) A left temporal
meningioma (51yrs, F). (B) The tumor stiffness map overlayed on the T2W image
suggests a stiff tumor. Edge-aware NNI outputs the stiffness values within the
tumor ROI. (C) The hyper-intense NOSS contour at the tumor boundary suggests a
mostly non-adherent interface. Areas without a clear contour (white arrow)
suggest an adherent interface locally. (D) Pattern recognition automatically
classifies the non-adherent (blue line) and adherent (white line) interfaces and
calculates the adhesion percentage over the 3D tumor surface (21.29% in
this case).
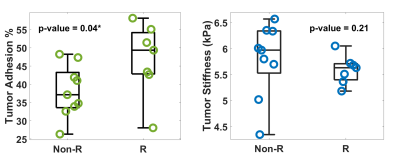
Fig.2. Group comparison of tumor adhesion and stiffness
between non-recurrent (Non-R) and recurrent (R) tumors with atypical features.
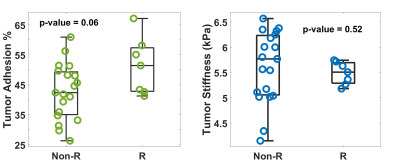
Fig.3. Group comparison of tumor adhesion and stiffness
between non-recurrent (Non-R) and recurrent (R) tumors with non-gross total
resection.
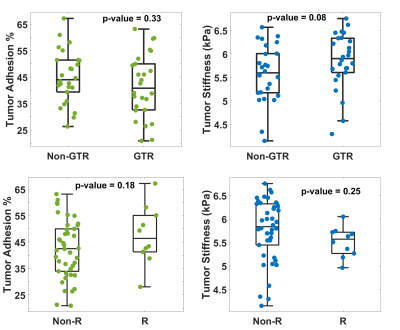
Fig.4. Group comparison of tumor adhesion and stiffness
between non-GTR and GTR (top) and non-recurrent (Non-R) and recurrent (R) (bottom)
for all tumors.
DOI: https://doi.org/10.58530/2023/0262