0260
An application of diffusion tensor image analysis along perivascular space (DTI-ALPS) in grading gliomas and predicting IDH1 mutation status
Hongquan Zhu1, Yuanhao Li1, Li Li1, Weiyin Vivian Liu2, and WenZhen Zhu1
1Department of Radiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 2GE Healthcare, MR Research China, Beijing, China
1Department of Radiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, 2GE Healthcare, MR Research China, Beijing, China
Synopsis
Keywords: Tumors, Brain, Glioma
Glioma could impair the glymphatic function with reduced CSF outflow. We aimed to use DTI-ALPS method to investigate the pathological change of glymphatic function in gliomas and evaluate its value in grading gliomas and predicting IDH1 mutation status. The lower-grade gliomas (LrGGs) showed higher ALPS Index than glioblastomas (GBMs). IDH1 wild-type group showed decreased ALPS Index compared with the IDH1 mutant group in LrGGs. But there was no difference between the IDH1 subtypes in GBMs. The ALPS Index showed good efficiency in grading gliomas and predicting IDH1 mutation status, it may be a potential imaging biomarker for diagnosing gliomas.Introduction and Purpose
Gliomas are the most common primary tumors in central nervous system1. Accurate and non-invasive understanding of tumor grade and molecular characters (especially IDH1 mutation status) before surgery is important for treatment and prognosis. Previous studies showed that glioma growth induced the damage of the glymphatic system and might further promote progression2,3. The diffusion tensor image analysis along perivascular space (DTI-ALPS) method was first used in Alzheimer's disease to evaluate glymphatic function4. We aimed to comprehensively investigate the difference of ALPS Index between lower-grade gliomas (LrGGs) and glioblastomas (GBMs) as well as between IDH1 wild-type gliomas and IDH1 mutant gliomas. In addition, the diagnostic efficacy of ALPS Index on grading tumor and detecting IDH1 mutation status was examined and compared with conventional diffusion kurtosis imaging (DKI) and diffusion tensor imaging (DTI) metrics.Methods
Eighty-one patients with pathologically confirmed gliomas were enrolled in the study. The data was acquired on a 3.0T MR scanner (Discovery MR750, GE Medical Systems, United States) with a 32-channel head coil. Diffusion kurtosis imaging (DKI) was using spin-echo echo-planar imaging sequence with 3b values (b = 0, 1250, and 2500 s/mm2) and 25 uniformly distributed directions for each nonzero b-value. Fractional anisotropy (FA), mean diffusivity (MD) and diffusion tensors along axes were computed using FSL; mean kurtosis (MK) was calculated using DIPY Toolbox. The ALPS Index metric was calculated at the level of lateral ventricle body using the following formula:$$ALPS\ Index=\frac{mean\left(D_{xproj}, D_{xassoc}\right)}{mean\left(D_{yproj}, D_{zassoc}\right)} \ \ \ \ \ \ (1)$$ An example of regions of interest (ROIs) placement was shown in Figure1. The comparisons of ALPS Index between LrGGs and GBMs as well as between IDH1-mutant group and IDH1 wild-type group in both LrGGs and GBMs were conducted by the analysis of covariance (ANCOVA) after controlling for age. In addition, diagnostic performance of each metric and combined metrics was assessed by receiver operating characteristic (ROC) curves. All statistical analyses were performed with SPSS (version21).Results
The ALPS Index, FA, MD and MK all showed significant differences between LrGGs and GBMs. Significantly higher ALPS Index and MD while significantly lower FA and MK in LrGGs than in GBMs (Figure 2). Moreover, all the metrics can differentiate IDH1 wild-type group and IDH1 mutant group in LrGGs but not in GBMs (Figure 3). Lower ALPS Index and MD while higher FA and MK in IDH1 wild-type LrGGs than IDH1 mutant LrGGs group. In differentiating LrGGs from GBMs, the ALPS Index alone showed the areas under the curve (AUC) of 0.774 while the combination of ALPS Index and MK showed the highest AUC of 0.864. In detecting IDH1 mutation status, the ALPS Index alone showed the AUC of 0.737 while the combination of ALPS Index and MD showed the highest AUC of 0.861 (Figure 4).Discussion
We demonstrated DTI-ALPS, FA, MD and MK had great potential in differentiation LrGGs from GBMs and also detecting IDH1 mutation status, partially consistent with previous study5. The LrGGs with higher ALPS Index suggested that the glymphatic function of LrGGs was better than that of GBMs. A rodent experiment has indicated that glioma could induce remodeling of glymphatic pathways6. In addition, the peritumoral edema formation may be related to glymphatic dysfunction and indirectly reflected insufficient clearance of interstitial fluid7. It is believed that the degree of peritumoral edema was correlated with AQP4 upregulation and AQP4 expression increases with higher glioma grades8. In other words, the difference of ALPS Index between LrGGs and GBMs may be correlated with extensive remolding of glymphatic pathway and the expression of AQP4, which needs further animal or human studies. The IDH1 wild-type LrGGs with lower ALPS Index suggested that wild-type IDH1 gene could induce more severe dysfunction of glymphatic system. Cheng et al.7 speculated that the lower glymphatic function of IDH1 wild-type LrGGs may be related to their higher aggressiveness and shorter duration of tumor growth, leading to uneasily remold the glymphatic pathway and compensate the function. Besides, IDH1 mutation increases the level of hypoxia-inducible factor −1α (HIF-1α) and upregulates the expression of vascular endothelial growth factor (VEGF)9. VEGF can enhance lymphangiogenesis in the meninges of the brain10. However, IDH1 wild-type gliomas with lower ALPS Index still needs further exploration. No significant differences of metrics in identifying IDH1 mutation status of GBMs, for GMBs might have complex microstructures, perfusions and other molecular changes besides IDH1 mutation. In ROC analyses, the ALPS Index showed equivalent efficiency to DTI, DKI derived metrics. Furthermore, the model of combined metrics improved the diagnostic performance for ALPS Index in reflection of additional pathophysiological information outside the tumor, and thus it complemented conventional DTI and DKI derived metrics. Last but not the least, this study showed DTI-ALPS had ability of detecting the pathological change of glymphatic system in gliomas.Conclusion
Our results indicated that the ALPS Index can effectively evaluate the glioma grades, predict IDH1 mutation status, and could further improve the diagnostic performance when combining conventional DTI and DKI derived metrics. The ALPS Index has great potential as a new imaging biomarker in glioma diagnosis.Acknowledgements
This project was supported by the National Natural Science Funds of China (Grants No.81730049).References
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-820.
- Ma Q, Schlegel F, Bachmann SB, et al. Lymphatic outflow of cerebrospinal fluid is reduced in glioma. Sci Rep 2019;9:14815.
- Lan YL, Wang H, Chen A, Zhang J. Update on the current knowledge of lymphatic drainage system and its emerging roles in glioma management. Immunology 2022.
- Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer's disease cases. Jpn J Radiol 2017;35:172-178.
- Zhao J, Wang YL, Li XB, et al. Comparative analysis of the diffusion kurtosis imaging and diffusion tensor imaging in grading gliomas, predicting tumour cell proliferation and IDH-1 gene mutation status. J Neurooncol 2019;141:195-203.
- Hu X, Deng Q, Ma L, et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res 2020;30:229-243.
- Toh CH, Siow TY. Factors Associated With Dysfunction of Glymphatic System in Patients With Glioma. Front Oncol 2021;11:744318.
- Mou K, Chen M, Mao Q, et al. AQP-4 in peritumoral edematous tissue is correlated with the degree of glioma and with expression of VEGF and HIF-alpha. J Neurooncol 2010;100:375-383.
- Huang J, Yu J, Tu L, Huang N, Li H, Luo Y. Isocitrate Dehydrogenase Mutations in Glioma: From Basic Discovery to Therapeutics Development. Front Oncol 2019;9:506.
- Song E, Mao T, Dong H, et al. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 2020;577:689-694.
Figures
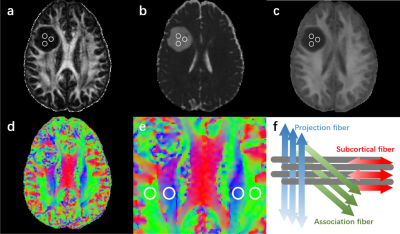
Figure 1. An example of regions of interest (ROIs) placement. (a-c) On FA, MD, MK maps, 3 ROIs were placed on the tumor parenchyma. (d-e) Color coded V1 maps illustrating ROIs on projection fiber (blue area) and association fiber (green area) in bilateral periventricular regions. (f) The schematic diagram showed the relationship between the direction of the perivascular space (gray cylinder) and the direction of the fibers.
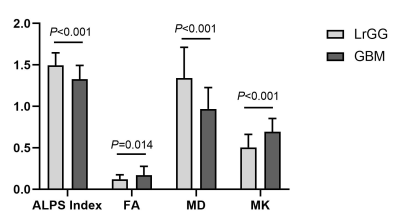
Figure 2. Bar charts of ALPS Index, FA, MD, and MK. The comparison between LrGGs and GBMs was anlyzed after controlling for age. MD are in units of 10–3 mm2/s.
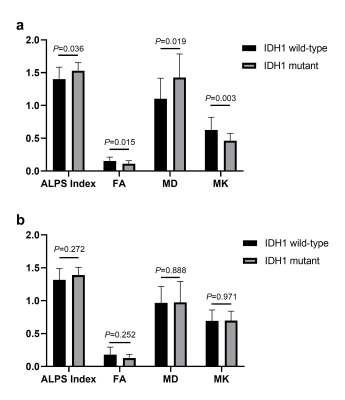
Figure 3. Bar charts for comparisons between IDH1 mutant and wild-type group in LrGGs (a) and GBMs (b) after controlling for age. MD are in units of 10–3 mm2/s.
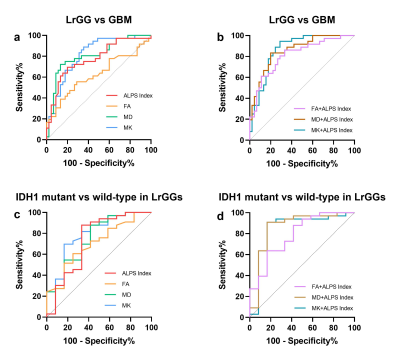
Figure 4. ROC curves of diffusion metrics in identifying tumor grades and IDH1 mutation status in LrGGs. ROC curves of (a, c) a single metric and (b, d) a combined model with two different diffusion metrics respectively in evaluating tumor grade and IDH1 mutation status in LrGGs.
DOI: https://doi.org/10.58530/2023/0260