0255
Preoperative Prediction of Brain Invasion in Meningiomas: A Radiomics Model based on Multimodal MRI and Quantitative Clinical Factors1Tianjin Huanhu Hospital, No.6 Jizhao Road, Jinnan District, Tianjin 300350, China., Tianjin, China, 2MR Collaboration, Siemens Healthineers Ltd., Beijing, China., Beijing, China
Synopsis
Keywords: Tumors, Radiomics, brain invasion
This study aimed to construct a radiomic model based on a large patient cohort to predict brain invasion (BI) in meningioma. By analyzing 97 patients with BI and 935 patients without BI, we found that the clinical risk factors for BI were male sex, tumor located at the skull base, and peritumoral edema volume. A binary logistic regression model combining these risk factors and multimodal MRI radiological characteristics was established. The constructed model achieved an excellent performance (AUC: 0.928) in terms of BI classification with an accuracy of 91.77%, which may be helpful for personalized treatment plan in meningioma.Introduction and Purpose
Brain invasion (BI), the invasive growth of meningioma in brain tissue, is one of the most important factors affecting the recurrence of meningioma1. BI has been added as an independent diagnostic standard for atypical meningioma (WHO Grade II) by the 2016 World Health Organization (WHO) classification of central nervous system tumors2, and also affects the treatment of patients. Increasing the number of meningioma tissue samples for pathological examination can significantly improve the detection rate of BI3. However, only a limited number of samples can be retained in the process of clinical surgical pathological sampling. It is of great significance to find a non-invasive method to evaluate the tumor mass and peritumorous tissue properties before surgery and accurately predict the BI of meningioma4. The purpose of our work is to investigate the relevant quantitative clinical and radiomics risk factors affecting brain invasion of meningioma and establish a radiomics model for non-invasive preoperative BI prediction, thus providing evidence for reducing misdiagnosis and formulating a personalized surgical plan.Methods
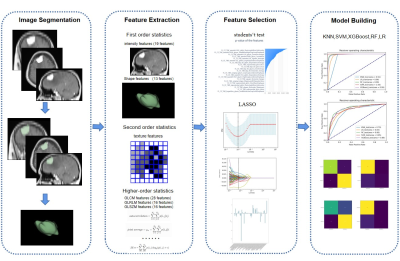
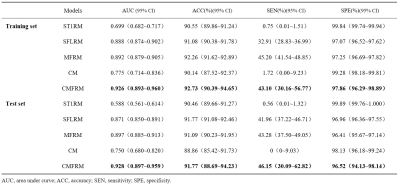
This study retrospectively included patients with pathology confirmed meningioma admitted from March 2016 to September 2021, whose clinical and imaging data were complete. The quantitative clinical risk factors related to BI, including age, sex, tumor volume, peritumoral brain edema (PTBE) volume, total lesion volume and edema index (EI), were determined by univariate and multivariate logistic regression analysis. Figure 1 showed the complete process for radiomics model construction. Radiologic features were extracted from the contrast-enhanced T1 weighted imaging (CE-T1WI) and contrast-enhanced fluid-attenuated inversion recovery (CE-FLAIR) sequences obtained on a 3T scanner (MAGNETOM Skyra & Trio A Tim, Siemens Healthcare, Erlangen, Germany) and standardized 5. Two sample t-test is used to select features related to BI, and the Least absolute shrinkage and selection operator (LASSO) model is used to filter features6. Multiple classifiers: K-Nearest Neighbor (KNN)7, Random Forest (RF)8, Logical Regression (LR)9, Support Vector Machines (SVM), eXtreme Gradient Boosting (XGBoost)10 are used to establish a single CE-T1WI radiomics model (Abbreviated to ST1RM), a single CE-FLAIR radiomics model(Abbreviated to SFLRM), CE-T1WI and CE-FLAIR multimodal MRI fusion radiomics models (Abbreviated to MFRM), quantitative clinical factor models (Abbreviated to CM), quantitative clinical factors and multimodal MRI fusion radiomics models (Abbreviated to CMFRM), using training and test datasets. To compare the performance of ST1RM, SFLRM, MFRM, CM and CMFRM model, area under the curve (AUC) of Receiver Operating Characteristic (ROC) was calculated and accuracy (ACC), sensitivity and specificity were reported.Results
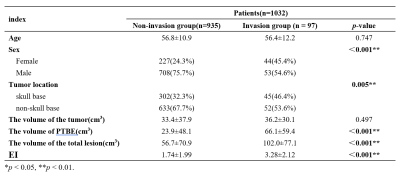
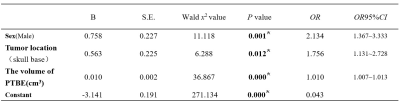
A total of 1032 patients (812 men and 299 women; mean age 56 years; range 5-86 years) were included by strict inclusion criteria, including 97 patients with BI and 935 patients without pathologically confirmed BI, shown in Table 1.Three factors were significantly correlated with BI (P < 0.05): male gender, tumor location at skull base and PTBE volume (cm3), which were shown in Table 2. There was no multiple collinearity (VIF<10).
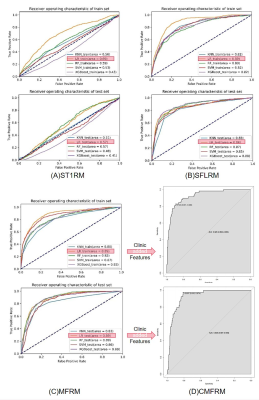
Compared with other classifiers, the model based on LR classifier has the best performance in ST1RM, SFLRM and MFRM. The AUC (0.897 [95% CI: 0.885~0.913]), accuracy (91.09% [95% CI: 90.23%~91.95%]), sensitivity (43.28%, 95% CI: [37.50%~49.05%]), specificity (96.41%, 95% CI: [95.67~97.14]) of MFRM using LR was better than ST1RM and SFLRM.
The CM, namely model only based on quantitative clinical factor, showed poor performance with AUC of 0.750(95%CI: 0.680~0.820).
As shown in Figure 2 and Table 3, the performance of CMFRM in test dataset (AUC: 0.928 [95% CI: 0.897~0.959], accuracy: 91.77% [95% CI: 88.69%~94.23%], sensitivity: 46.15% [95% CI: 30.09%~62.82%], specificity: 96.52% [95% CI: 94.13%~98.14%]) was better than all other models.
Discussion and Conclusions
This study established and compared the performance of different radiomics models constructed based on preoperative quantitative clinical factors and multimodal MRI in the prediction of brain invasion using a large group of cases with pathological examination as the gold standard. It’s found that the quantitative clinical factors and multimodal MRI fusion radiomics model has the best performance than other models. This work firstly investigated the relevant quantitative clinical risk factors associated with BI in a large cohort, which was seldomly performed in previous studies. Furthermore, based on multi-modal MR Images (CE-T1WI and CE-FLAIR), stable and interpretable radiomics features were selected and a stable radiomics model was established. It’s found that the CE-FLAIR act as an important role in the BI prediction. We further compared the performance of different classifiers and found that LR classifier achieved the best performance. This study finally established and validate a model based on both quantitative clinical factors and multimodal MRI features, and the model achieved excellent performance in BI prediction, indicating that the established model can be used for effective prediction of BI in meningioma patients, thus providing evidence for reducing misdiagnosis and formulating a personalized surgical plan.Acknowledgements
We sincerely thank the participants in this study.
This work was supported by Tianjin Natural Science Foundation (Study on the MRI Prediction of brain invasion and grading of meningioma based on Multimodal Deep Learning 20JCYBJC00960)
References
1.Morokoff, A.P., J. Zauberman, and P.M. Black, Surgery for convexity meningiomas. Neurosurgery, 2008. 63(3): p. 427-33; discussion 433-4.
2.Louis, D.N., et al., The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol, 2016. 131(6): p. 803-20.
3.Pizem, J., et al., Brain invasion assessability in meningiomas is related to meningioma size and grade, and can be improved by extensive sampling of the surgically removed meningioma specimen. Clin Neuropathol, 2014. 33(5): p. 354-63.
4.Nowosielski, M., et al., Diagnostic challenges in meningioma. Neuro Oncol, 2017. 19(12): p. 1588-1598.
5.Lambin, P., et al., Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer, 2012. 48(4): p. 441-6.
6.Tibshirani, R., Regression shrinkage and selection via the lasso: a retrospective. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 2011. 73(3): p. 273-282.
7.Cover, T. and P. Hart, Nearest neighbor pattern classification. IEEE Transactions on Information Theory, 1967. 13(1): p. 21-27.
8.Zhang, C. and Y. Ma, Ensemble machine learning: methods and applications. 2012: Springer.
9.LaValley, M.P., Logistic Regression. Circulation, 2008. 117(18): p. 2395-2399.
10.Chen, T., et al., Xgboost: extreme gradient boosting. 2015. 1(4): p. 1-4.
Figures