0254
Comparison of radio-pathomic maps of tumor probability to 5-ALA guided surgical resection cavities in glioblastoma patients
Samuel A Bobholz1, Allison K Lowman1, Savannah R Duenweg2, Aleksandra Winiarz2, Margaret Stebbins2, Fitzgerald Kyereme1, Jennifer Connelly3, Dylan Coss4, Wade M Mueller5, Mohit Agarwal1, Anjishnu Banerjee6, Max Krucoff5, and Peter S LaViolette1,7
1Radiology, Medical College of Wisconsin, Milwaukee, WI, United States, 2Biophysics, Medical College of Wisconsin, Milwaukee, WI, United States, 3Neurology, Medical College of Wisconsin, Milwaukee, WI, United States, 4Pathology, Medical College of Wisconsin, Milwaukee, WI, United States, 5Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States, 6Biostatistics, Medical College of Wisconsin, Milwaukee, WI, United States, 7Biomedical Engineering, Medical College of Wisconsin, Milwaukee, WI, United States
1Radiology, Medical College of Wisconsin, Milwaukee, WI, United States, 2Biophysics, Medical College of Wisconsin, Milwaukee, WI, United States, 3Neurology, Medical College of Wisconsin, Milwaukee, WI, United States, 4Pathology, Medical College of Wisconsin, Milwaukee, WI, United States, 5Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States, 6Biostatistics, Medical College of Wisconsin, Milwaukee, WI, United States, 7Biomedical Engineering, Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Keywords: Tumors, Brain
This study sought to compare previously published autopsy-based radio-pathomic maps of pre-surgical cellularity and tumor probability to beyond-contrast 5-ALA guided resection margins to assess concordance in identifying non-enhancing tumor between the techniques. In a series of 10 cases, radio-pathomic maps were able to identify areas of non-enhancing tumor prior to surgery that coincided with either 5-ALA guided resection margins or areas of future recurrence in 7 of 10 cases, suggesting that highlighted areas on these maps indicate active, progressive tumor.Introduction
Surgical removal is a critical component in the current standard of care for glioblastomas, where maximal safe resection shows strong associations with patient survival. The surgically resected margin is typically defined using gadolinium-enhanced areas on T1-weighted imaging, which is known to underestimate the true tumor margin in both de novo and recurrent cases. Fluorescence-guided surgery using 5-aminolevulinic acid (5-ALA) has allowed surgeons to expand the resection margin beyond signatures dependent on blood-brain barrier disruption, but the technique is limited to what is readily visible during the intervention (Figure 1). Therefore, this study sought to compare previously published autopsy-based radio-pathomic maps of pre-surgical cellularity and tumor probability to beyond-contrast 5-ALA guided resection margins to assess concordance in identifying non-enhancing tumor between the techniques.Methods
This study includes 10 glioblastoma patients that underwent 5-ALA guided surgery with pre- and post-surgical imaging and confirmed resection beyond initial T1 contrast enhancement. Radio-pathomic maps were generated for each pre-surgical timepoint using our previously published technique1,2. Briefly, T1, T1C, FLAIR, and ADC images were used as features to predict cellularity, extracellular fluid density, cytoplasm density, and tumor probability using autopsy tissue samples aligned to the last clinical imaging prior to death as ground truth (Figure 2). A training dataset of 43 patients was used to train a bagging regression ensemble using 5 by 5 voxel tiles from the MRI as input and voxel-wise pathological characteristics as labels, with a held-out test set of 22 subjects used for model validation. These models demonstrated good quantitative performance and readily identified pathologically-validated areas of occult tumor invasion. Pre-surgical cellularity and tumor probability maps were then qualitatively compared to the surgical resection margin to identify whether the non-invasive maps would be able to capture the extent of non-enhancing invasion. Imaging available from a subset of patients with suspected recurrence following surgery were also qualitatively compared to the radio-pathomic maps to identify cases where 5-ALA-guided resection may have underestimated the true tumor burden.Results
Figure 3 shows five cases where cellularity and tumor probability maps identify tumor infiltration beyond initial contrast enhancement that coincide with the 5-ALA resection margin. For a subset of these cases, the hypercellular/high tumor likelihood region extends beyond the resection cavity, indicating areas where 5-ALA may have failed to encompass the full extent of non-enhancing invasion. This is supported by the findings from two cases with available recurrence data in Figure 4, where the radio-pathomic maps identify large areas of tumor well-beyond both initial contrast enhancement and the surgical cavity, but encompass areas where patients exhibit recurrence following surgery, indicating areas of active occult tumor spared treatment. Figure 5 shows three cases where the radio-pathomic maps failed to fully capture the extent of non-enhancing tumor as defined by the resection cavity, though some subjects show subtle, non-specific indications of abnormal pathology in these missed regions.Discussion
In a series of 10 cases, radio-pathomic maps were able to identify areas of non-enhancing tumor prior to surgery that coincided with either 5-ALA guided resection margins or areas of future recurrence in 7 of 10 cases, suggesting that highlighted areas on these maps indicate active, progressive tumor. By non-invasively identifying these regions on pre-surgical imaging, surgical planning may be able to consider these more distant regions of proliferative tumor and surgical margins may be able to expand to encompass an even greater portion of tumor than using 5-ALA alone. Due to 5-ALA requiring exposed tumor surface for visual identification, these maps could direct surgeons towards fluorescing areas beyond what is visible in the immediate environment of the initial enhancement. While radio-pathomic maps failed to capture the full extent of resection in 3 cases, 5-ALA is known to stain false positives in areas of infection or radiation necrosis, such that using a consensus measure between 5-ALA positivity and high radio-pathomic tumor probability may provide greater clinical confidence than either measure alone. Future research comparing biopsy cores collected from the consensus region between 5-ALA and tumor probability maps is essential to providing quantitative accuracy of the combined technique, as well as future research investigating the chance of recurrence in distant areas of high predicted tumor probability.Acknowledgements
No acknowledgement found.References
1. Radio-Pathomic Maps of Cell Density Identify Brain Tumor Invasion beyond Traditional MRI-Defined Margins, S.A. Bobholz, A.K. Lowman, M. Brehler, F. Kyereme, S.R. Duenweg, J. Sherman, S.D. McGarry, E.J. Cochran, J. Connelly, W.M. Mueller, M. Agarwal, A. Banerjee, P.S. LaViolette, American Journal of Neuroradiology Apr 2022, DOI: 10.3174/ajnr.A7477
2. Bobholz, S. A., Lowman, A. K., Connelly, J. M., Duenweg, S. R., Winiarz, A., Brehler, M., … LaViolette, P. S. (2022). Non-invasive tumor probability maps developed using autopsy tissue identify novel areas of tumor beyond the imaging-defined margin. MedRxiv, 2022.08.17.22278910. https://doi.org/10.1101/2022.08.17.22278910
Figures
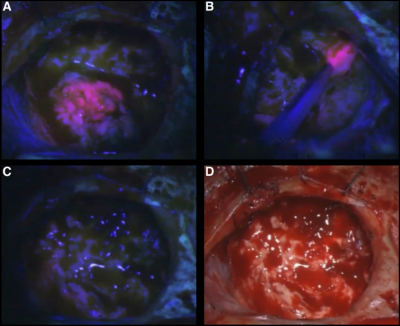
Images of resection cavity during 5-ALA-guided surgery. Bright pink fluorescence indicates tumor. A) Primary resected tumor mass. B) Additional fluorescence found at resection margin after tumor was removed. C) Final resection cavity under fluorescence and D) white light.

Overview of tumor probability map generation. MRI-based models (input: 5 by 5 tiles from T1, T1C, FLAIR and ADC) are used to predict segmented cellularity, extracellular fluid, and cytoplasm density using aligned autopsy tissue samples as ground truth. A second model uses tissue segmentations to predict pathologist-confirmed tumor presence in histology space, which is then combined with the tissue maps from the MRI-based model to generate whole-brain, non-invasive tumor probability maps. See references for further detail.
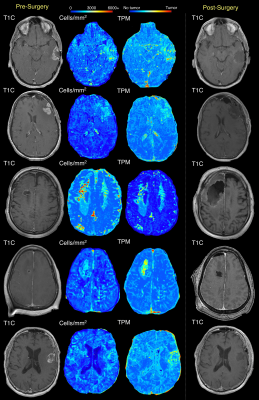
Examples of subjects where pre-surgical tumor probability maps identify non-enhancing portions of the 5-ALA guided resection cavity. A subset of patients show predicted infiltration beyond the resection cavity, which may indicate further tumor invasion.
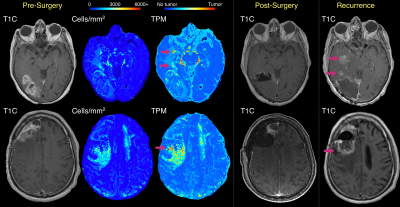
Examples of subjects with predicted tumor infiltration beyond the 5-ALA guided resection cavity, coupled with T1C scans at tumor recurrence. In both cases, the predicted tumor region missed by 5-ALA guided surgery encompasses the region where the tumor recurs, indicating that these regions may progress into mature tumor when spared treatment.
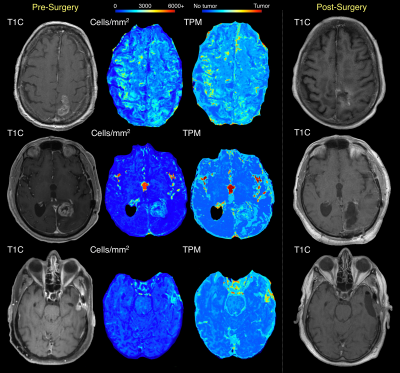
Examples of subjects where predicted tumor infiltration fails to distinguish the full extent of non-enhancing tumor removed during 5-ALA guided surgery.
DOI: https://doi.org/10.58530/2023/0254