0252
Ultrafast multi-parametric quantitative MRI using Multi-TR echo-planar time-resolved imaging (Multi-TR-EPTI)1School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China
Synopsis
Keywords: Data Acquisition, Data Acquisition
Echo planar time-resolved imaging (EPTI) is a recently developed multi-shot EPI method that provides time-resolved multi-echo images and providing multi-contrast images. Based on this basis, here we present Multi-TR-EPTI, a technique developed to achieve distortion-free and Multi-parametric quantitative MRI including T1, T2, T2*, and PD mapping. Multi-TR-EPTI exploits an optimized spatiotemporal CAIPI encoding in the k-TE-TR space.which can recover the k-TE-TR space within the same shot number as the original EPTI. A subspace reconstruction was employed to obtain hundreds of high-quality multi-contrast images at sub-millisecond temporal increments. Multi-TR-EPTI adopts different TRs to provide T1 contrast while improving scan efficiency.Introduction
The time-resolved imaging method of EPTI[1] allows image recovery at each echo time point throughout the readout to provides time-resolved multi-echo images. However, EPTI can be further developed. EPTI uniformly undersamples in k-space, while different roles exist for the low and high frequency signals in k-space and the TR between each excitation scan is fixed, so that EPTI cannot provide T1 contrast. Some methods have been proposed to optimize EPTI. Dong proposed ACE-EPTI[2] to optimize the EPTI sampling trajectory, using three shots to acquire k-space low-frequency and high-frequency signals, as well as more intensive acquisition in the center of k-space and in k-space where the signal is stronger at earlier times. 3D-EPTI[3] exploit the spatiotemporal correlation of MRI data at multiple timescales through new encoding strategies within and between efficient continuous readouts to provide robust and repeatable whole-brain isotropic resolution. vFA-EPTI[4] provide a the complementary k-t encoding strategy which is further extended across flip angles to improve the performance. Here, we propose Multi-TR-EPTI based on EPTI by optimizing the design of the spatio-temporal encoding sampling scheme and applying the spatio-temporal encoding approach of ACE-EPTI to the k-TE-TR space. A subspace reconstruction based on locally low-rank regularization (LLR) [5-7] was employed to obtain multi-contrast images. Multi-TR-EPTI can improve scanning efficiency while providing distortion free multi-contrast images with T1, T2, T2*, PD and B0 mapping.Methods
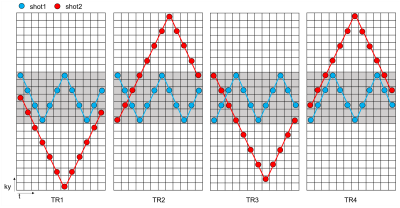
The space-time coding trajectory of Muliti-TR-EPTI is shown in Figure 1 below:1) Only two shots are needed for each TR's k-space, for low(gray filed in Fig.1) and high frequencies respectively. 2) The k-space acquisition trajectories of different TRs are oriented differently, with complementary phase-encoded sampling along t dimension to control aliasing. 3) More intensive sampling at the center of k-space and at start time allow improving SNR and more accurate LLR constrained reconstruction. 4) Reduced ky blip between adjacent time points to shorten ESP and alleviate potential eddy current effects. 5) Low frequency signals obtained from navigation acquisition can be used for phase variation estimation between shot and shot.In-vivo experiments was performed on Siemens Aera 1.5T system with ethical approval. We first acquired fully sampled k-space data using EPSI with different TRs as ground truth, and then applied the sampling trajectory of Multi-TR-EPTI on EPSI data to obtain the corresponding undersampled data. Parameters of EPSI acquisition include four TRs (3000ms,1500ms,1000ms and 500ms), TE=150ms, FOV =240×240x4mm3, matrix size=224×220,120 echoes. Multi-TR-EPTI keeps consistent with EPSI parameters with a change of 4 ky between adjacent time points.
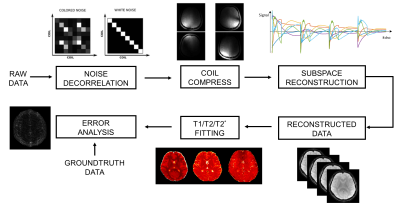
The reconstruction pipeline is shown in Figure. 2. Even odd ghost correction with inline three-echo navigations, SVD-based coil compression[8] and coil sensitivity estimation are done in the pre-processing step using low-resolution fully sampled data before reconstruction. Then the full k-TE-TR k space is reconstructed using the LLR-based reconstruction algorithm. Finally, we perform error analysis and quantitative analysis such as T1/T2/T2* fitting on the reconstruction results. In the GE-SE sequence, the TR dimension follows the T1 relaxation recovery, whose signal evolution at start time is as follows:
$$S_0(\mathrm{TR})=N(H)\left[1-2 \mathrm{e}^{-\frac{T R-\frac{T E}{2}}{T_1}}+\mathrm{e}^{-\frac{T R}{T_1}}\right]$$
which can be used for T1 fitting. The BM4D[9] denoising algorithm is used before quantitative mapping. However, it is worth to mention that the relaxation basis and the fitting model may be different for retrospective undersampling from fully sampled multi-TR EPSI compared to actual undersampled multi-TR-EPTI. 3D-EPTI-based acquisition may be able to be incorporated to keep the relaxation basis and fitting model the same. More related work need be carried out in the further study.
Results and Discussion
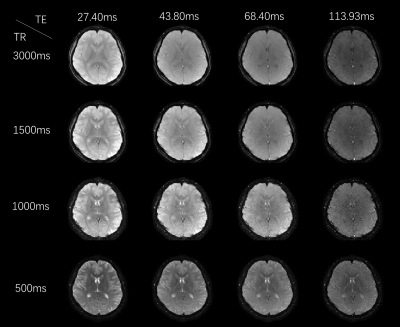
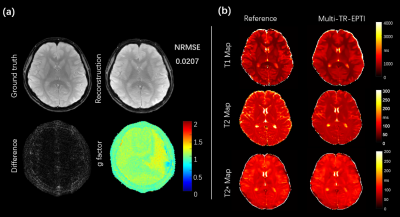
Figure 3 shows the reconstruction results of Multi-TR-EPTI with 4 different TRs and different TEs. Significant contrast change are presented in the reconstructed images for different TRs. Tissues with long T1 recover slowly and those with short T1 recover quickly, thereby leading to signal intensity variation and constrast change. The results of 4TR at the same ehco shows T1 relaxation curve and the results of 4 TE of the same TR shows T2 relaxation curve.Figure 4a shows the quantitative error analysis of the innovative Multi-TR-EPTI. Taking the 15th echo at TR=3s as an example, the calculated error map is shown in the figure compared with the ground truth of full sampling, and the resulting NRMSE is 0.0207. The g-factor map of the subspace reconstruction algorithm based on LLR is shown in the figure. The error map and g-factor demonstrate the robustness and reliability of the subspace reconstruction algorithm.
Figure 4b shows quantitative mapping results. The left column is the reference given by the vendor provided sequence. The right column shows the quantitative map obtained by fitting from the Multi-TR-EPTI reconstruction results. Figure 4 shows that fitting results of the quantitative map are basically consistent with the reference T1, T2, T2* maps.
Conclusions
Multi-TR-EPTI can provide distortion free multi-contrast images with T1, T2, T2*, PD and B0 mapping. Improvements in scanning efficiency will be investigated in future study.Acknowledgements
This work is supported by the National Natural Science Foundation of China National Science Foundation of China (No. 62001290), Shanghai Science and Technology Development Funds (21DZ1100300), and Shanghai Sailing Program (20YF1420900), and sponsored by the National Science and Technology Innovation 2030 Major Project (2022ZD0208601).References
1.Wang, F.Y.X., et al., Echo planar time-resolved imaging (EPTI). Magnetic Resonance in Medicine, 2019. 81(6): p. 3599-3615.
2.Dong, Z.J., et al., SNR-efficient distortion-free diffusion relaxometry imaging using accelerated echo-train shifted echo-planar time-resolving imaging (ACE-EPTI). Magnetic Resonance in Medicine, 2022.
3.Wang, F.Y.X., et al., 3D Echo Planar Time-resolved Imaging (3D-EPTI) for ultrafast multi-parametric quantitative MRI. Neuroimage, 2022. 250.
4.Dong, Z., et al., Variable flip angle echo planar time-resolved imaging (vFA-EPTI) for fast high-resolution gradient echo myelin water imaging. NeuroImage, 2021. 232: p. 117897.
5.Zhang, T., J.M. Pauly, and I.R. Levesque, Accelerating Parameter Mapping with a Locally Low Rank Constraint. Magnetic Resonance in Medicine, 2015. 73(2): p. 655-661.
6.Tamir, J.I., et al. T1-T2 shuffling: multi-contrast 3D fast spin-echo with T1 and T2 sensitivity. in ISMRM 25th Annual Meeting & Exhibition (Honolulu, HI, 22–24 April 2017). 2017.
7.Tamir, J., et al., T2-Shuff-LL: Multi-contrast 3D Shuffling Combining Fast Spin-Echo and Look-Locker Gradient Echo.
8.Zhang, T., et al., Coil compression for accelerated imaging with Cartesian sampling. Magnetic Resonance in Medicine, 2013. 69(2): p. 571-582.
9.Maggioni, M., et al., Nonlocal Transform-Domain Filter for Volumetric Data Denoising and Reconstruction. Ieee Transactions on Image Processing, 2013. 22(1): p. 119-133.
Figures